
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
Chapter 11
Heterokontophyta
SYNUROPHYCEAE
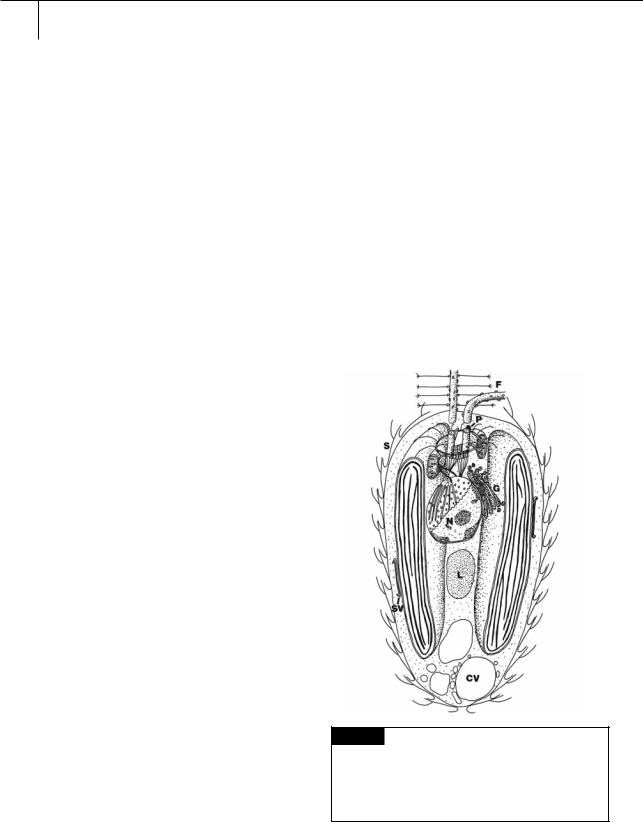
The Synurophyceae are closely related to the Chrysophyceae (Ariztia et al., 1991). The Synurophyceae differ, however, from the Chrysophyceae in the following: the Synurophyceae have chlorophylls a and c1 (Andersen and Mulkey, 1983), the flagella are inserted into the cell approximately parallel to one another (Fig. 11.1), there is a photoreceptor near the base of each flagellum, there is no eyespot, and the contractile vacuole is in the posterior portion of the cell (Lavau et al., 1997; Andersen et al., 1999). Chloroplast endoplasmic reticulum is present in a few species, but absent in most. The cells usually are covered by bilaterally symmetrical scales.
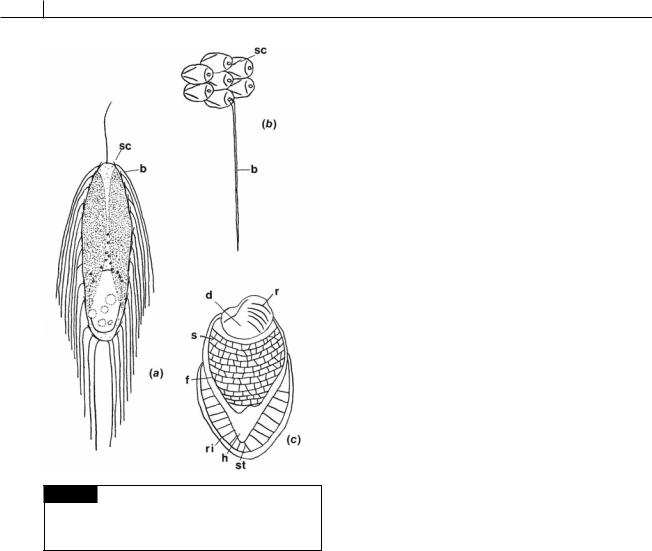
In the Synurophyceae, scales composed of silica commonly occur outside the cell (Figs. 11.1, 11.2). The scales are bilaterally symmetrical and are formed in a silica deposition vesicle. The membrane of the silica deposition vesicle (the silicalemma) controls the shape of the scale along with proteins and glycoproteins that adhere the developing scale to the silicalemma (Schultz et al., 2001). The presence of germanium in the medium results in inhibition of scale formation (Klaveness and Guillard, 1975). The scales are carried in the scale vesicle to the plasma membrane where the plasma membrane and the scale vesicle fuse, releasing the scales outside the cell (Beech et al., 1990). The scales are held next to the cell in an organic envelope (Ludwig et al., 1996), which is either hyaline or yellow-brown, the latter appearance being due to the impregnation of iron salts. The scales of the Synurophyceae are commonly
composed of a number of parts, such as the dome, shield, and bristle of Mallomonas (Lavau and Wetherbee, 1994) (Fig. 11.2). The scales of the Synurophyceae are overlapped precisely so that the anterior end of one scale overlaps the right margin of the scale to its left (Leadbeater, 1990).
Fig. 11.1 Semidiagrammatic drawing of the cytology of
Synura, showing the characteristic cytology of the Synurophyceae. (CV) Contractile vacuole; (F) flagella;
(G) Golgi; (L) chrysolaminarin vesicle; (N) nucleus;
(P) photoreceptor; (S) scale; (SV) scale vesicle. (Adapted from Andersen, 1985.)
350 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 11.2 Mallomonas zellensis. (a) Whole cell. (b) Scales. (c) A scale with the bristle removed. (b) Bristle; (d) dome;
(f) flange; (h) hood; (r) ribs; (ri) rim; (s) shield; (sc) scale; (st) strut. (After Fott, 1962.)
The scales are cemented together to form a scale case by the organic envelope (Fig. 11.4). This precise arrangement of scales differs from the loosely arranged scales of the Chrysophyceae.
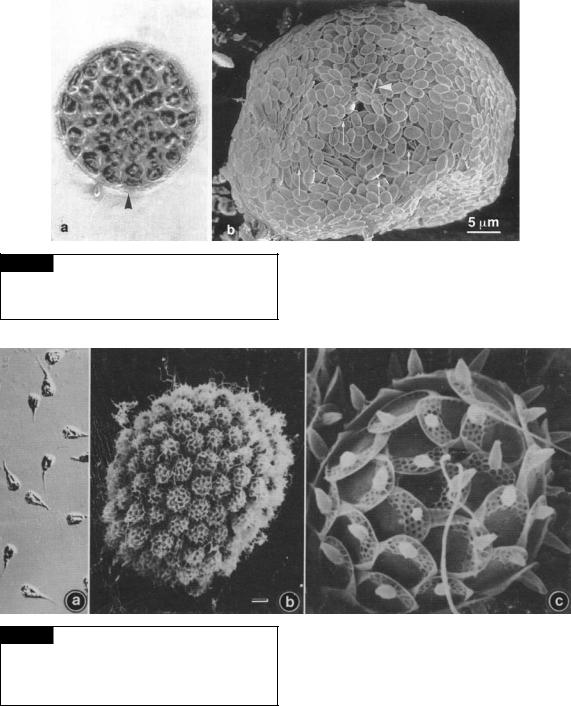
Tessellaria volvocina (Fig. 11.3) is a colonial member of the class that has a large number of cells encased within a multilayer covering of siliceous scales. The individual cells of T. volvocina do not have a covering of scales (Tyler et al., 1989; Pipes and Leedale, 1992).
Analysis of lake sediments often reveals the presence of the silicified scales of the Synurophyceae as well as the silicified frustules of diatoms (Smol et al., 1984; Dixit et al., 1999). Analysis of the species that produced these silicified deposits can often result in a history of
environmental conditions in the lake. Diatoms usually do not grow in waters below a pH of 5.8 to 6, whereas several members of the Synurophyceae thrive in acidic lakes (Saxby-Rouen et al., 1997). As environmental concerns over the acidification of lakes by acid rains increase, these species will probably be more widely used as indicators of lake acidification.
Unlike the Chrysophyceae, the Synurophyceae are not phagocytotic, probably because the fixed scale-case is solidly formed and bacteria and food particles cannot easily reach the cell membrane. The cysts (statospores or stromatocysts) of the Synurophyceae are usually more elaborate than those of the Chrysophyceae.
Classification
The Synurophyceae share affinities with the Bacillariophyceae, Chrysophyceae, and Phaeophyceae (Lavau et al., 1997; Andersen et al., 1999). Because of their silicified outer covering, they have been called “flagellated diatoms”; yet they have many of the characteristics of the Chrysophyceae such as statospores.
There is a single class, the Synurales. All of the organisms are flagellates. Siphonaceous forms do not exist, as true filaments cannot be formed by organisms with a silica scale-case, and coccoid forms would be similar to diatoms. The diatoms
Corethron, Rhizosolenia, and Attheya are similar in appearance to coccoid Synurophyceae (Andersen, 1987).
Synura (Fig. 11.4) is a widely distributed colonial member of the class. The life cycle of Synura petersenii (Figs. 11.5(a), 11.6) has been described by Sandgren and Flanagin (1986). Vegetative colonies of S. petersenii normally consist of a number of biflagellate cells joined at their posterior end. The cells are covered with silicified scales (Leadbeater, 1990). Each cell has two parietal chloroplasts, a central nucleus, and a large posterior chrysolamarin vacuole. Sexual reproduction in S. petersenii is isogamous and heterothallic. Gametes appear when two compatible clones are mixed. No special culture conditions are required to induce sexual behavior. Actively growing cell populations are continually
HETEROKONTOPHYTA, SYNUROPHYCEAE |
351 |
|
|
Fig. 11.3 Tessellaria volvocina. (a) Light micrograph. Arrowhead points to a scale case. (b) Scanning electron micrograph showing overlapping of plate scales and rare spine scales (arrowhead). (From Tyler et al., 1989.)
Fig. 11.4 Synura uvella. (a) Light micrograph. (b) Scanning electron micrograph of a colony of cells. (c) Scanning electron micrograph of a single cell showing the two flagella emerging from an apical pore in the scale case. Bar 10 m. (From Andersen, 1985.)
receptive to mating when mixed with a sufficient number of cells from a compatible clone. Male gametes are solitary biflagellate cells that appear similar to vegetative cells. The solitary male
gametes are probably released from the colonies of the male clone. The female gametes occur in colonies or as solitary cells, and are also similar in appearance to vegetative cells. Male and female gametes initially contact each other anteriorly; this is followed by lateral fusion. The silica scale layers mingle so that the resulting fusion cell maintains a complete scale layer. The biflagellate planozygote undergoes minor shape changes

352 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 11.5 Scanning electron micrographs. (a) Synura petersenii, whole colony. (b) S. echinulata. (c) Mallomonas acaroides, whole cell. (d) M. acaroides, distal end of helmet bristles. Bar 1 m. (From Dürrschmidt, 1984.)
|
|
|
|
|
|
|
|
|
|
|
|
Fig. 11.6 |
Process of sexual copulation and zygotic |
|
|
(a) |
|
|
|
|
|
|
|
|
|
encystment in Synura petersenii. (a) Initial contact of a solitary |
|
|
|
|
|
|
|
|
|
|
|
|
|
male gamete with a female gamete within a colony. (b) An |
|
|
|
|
|
|
|
(b) |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
early stage in plasmogamy characterized by the lateral fusion |
|||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
of cells, each with active flagellar pairs. (c) A biflagellate |
|
|
|
|
|
|
|
|
|
|
|
|
|
planozygote stage with the fusion cell containing four plastids, |
|
|
|
|
|
|
|
|
|
|
|
|
|
two nuclei, and two chrysolaminarin vesicles. Note that the |
|
|
|
|
|
|
|
|
|
|
|
|
|
planozygote is completely covered with scales. (d) An early |
|
|
|
|
|
|
|
|
|
|
|
|
|
stage in zygotic encystment during which the remaining flagella |
|
|
|
|
|
|
|
|
|
|
|
|
|
are lost and significant rearrangement of cytoplasmic |
|
|
|
|
|
|
|
|
|
|
|
|
|
organelles occurs to establish a symmetrical orientation. |
|
|
|
|
|
|
|
|
|
|
|
(c) |
|
||
|
|
|
|
|
|
|
|
|
|
|
Fusion of chrysolaminarin vesicles occurs, and the nuclei |
||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
become associated with the inner margins of the plastids. (e) |
|
|
|
|
|
|
|
|
|
|
|
|
|
A mature zygotic statospore as it ultimately appears taking the |
|
|
|
|
|
|
|
|
(d) |
|
|
|
place of the female gamete in the colony. (f) Germination of |
||
(f) |
|
|
|
|
|
|
|
|
|
||||
|
|
|
(e) |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
statospore. (From Sandgren and Flanagin, 1986.) |
||||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
