
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
CHLOROPHYTA 173
Fig. 5.35 (a) Chaetomorpha aerea.
(b) Cladophora microcladioides. (After
Smith, 1969.)
its ability to quickly respond to enhanced nutrient supply, Ulva has proliferated in many areas that have received anthropogenic (related to man) nutrient enrichment. A feature of nuisance growths of Ulva in enclosed and semienclosed waters is that Ulva comprises a large proportion of drift plants, which may smother other benthic communities or be cast ashore where they decompose, causing considerable aesthetic nuisance (Blomster et al., 2002; Nelson et al., 2003; Kim et al., 2004).
Ulva is commonly known as the sea lettuce or green laver, and has been eaten as a salad or used in soups (Chen, 1998). The chemical composition of dried U. latuca is 15% protein, 50% sugar and starch, less than 1% fat, and 11% water, making it usable as roughage in the human digestive system. In World War I, Phillipsen prepared a salad with U. latuca, and Monostroma, which he flavored with salad cream, vinegar, lemon, pepper, onions, and oil, and which he described as “wonderfully nice, slightly piquant and not inferior to the best garden salad” although one wonders after adding all of his condiments whether
he was able to appreciate anything about seaweeds. The eminent French algologist Savaugeau prepared such a salad without condiments and said that “it was leathery and waxy in taste, and in spite of a good digestion I thought I would be ill” (Chapman, 1970).
Cladophorales
The filamentous genera in this order have multinucleate cells, usually with a parietal or reticulate chloroplast. The filaments may be branched or unbranched. The reticulate chloroplast has pyrenoids at the intersections of the reticulum.
Cladophora (Fig. 5.35(b)) and Chaetomorpha (Figs. 5.35(a), 5.36), each with an isomorphic alternation of generations, are common members of this order. Cladophora, found in freshwater and marine habitats, may be the most ubiquitous macroalga in freshwaters worldwide (Dodds and Gudder, 1992). This filamentous alga can reach nuisance levels as a result of cultural eutrophication. Cladophora is predominantly benthic, and is often found in the region of unidirectional flow or in periodic wave action. In freshwater, it is a midto late-succession

174 EVOLUTION OF THE CHLOROPLAST
Fig. 5.36 Chaetomorpha aerea showing the process by which a new filament is produced from cell contents after disruption of the filament. (a) Vegetative filament.
(b) Protoplasm is expelled from damaged cells and spreads in seawater. (c) Extruded cell organelles aggregate in seawater. (d) Regenerated protoplast forms an envelope within 10 minutes after wounding. (From Klotchkova
et al., 2003.)
species. Cladophora is colonized by a wide variety of epiphytes because it offers a substrate that is anchored against flow disturbance.
Dasycladales
The plants in this order are all tropical and subtropical marine plants, most of them calcified. The members of the order are a clearly defined group having the following characteristics:
(1) radial symmetry with an erect axis bearing branches; (2) uninucleate vegetative thallus, with a multinucleate condition developing just before reproduction; (3) gametes formed in operculate cysts within specialized gametangia.
The coenocytic algae in the Dasycladales and Caulerpales respond to injury by rapidly forming gel-like wound plugs, thereby preventing loss of cytoplasm. The wound plugs are formed from extruded cytoplasm that forms the plug through interaction of carbohydrates and lectins (Ross et al., 2005). A new cell wall is formed under the gelatinous plug.
The Dasycladales has a paleontological record that extends back to the Precambrian–Cambrian boundary (ca. 570 million years ago) (Berger and Kaever, 1992). Of the 175 known fossil genera, only 11 are extant. The Dasycladales are in fact “living fossils.” As defined by Stanley (1979), living fossils are organisms that include extant clades that have survived for long intervals of geological time at low numerical diversity and exhibit primitive morphological characteristics that have undergone little evolutionary change.
There are two families within the order, the first of which is extinct:
CHLOROPHYTA 175
Family 1 Receptaculitaceae: laterals produced spirally on erect axis; relatively large plants; all extinct.
Family 2 Dasycladaceae: laterals produced in whorls on erect axis; relatively small plants; extinct and extant.
Receptaculitaceae
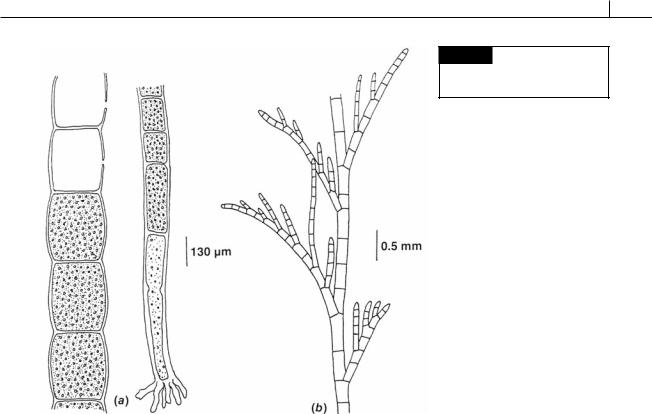
The plants in this order existed from the Lower Ordovician to the Permo-Carboniferous Period. They were non-septate marine dasycladaceous algae shaped like a light bulb, with the upper portion consisting of a large number of spiral laterals attached to the main axis. They were calcified, with calcification being heavier around the periphery of the thallus. Ischadites abbottae was a Silurian alga that grew in shallow reef water. The thallus was globose, formed by spirally attached laterals with the largest laterals in the mid latitudes (Fig. 5.37). The upper laterals gradually became wider toward the periphery of the plant, then suddenly expanded into heads at the periphery. The main axis and laterals were calcified (Nitecki, 1971).
Dasycladaceae
This family is characterized by a thallus having laterals arranged in whorls on the main axis. There are a number of different algae in the family (Zechman, 2003), with Acetabularia (Figs. 5.38, 5.40, 5.41) being the best known (Mandoli, 1998). At maturity Acetabularia has a naked axis with a single gametangial disc at the apex. In contrast, the axes of Dasycladus (Fig. 5.39(a)) and Neomeris (Fig. 5.39(b)) are enclosed in whorls of laterals that form a fairly solid cortication.
The first fossils of this family appeared about the Middle Silurian, and some of the extant genera are well represented in the fossil record, Neomeris going back to the Cretaceous and Acetabularia to the Tertiary (Johnson, 1961).
The life cycle of Acetabularia (Fig. 5.41) is similar to other plants in the order. Acetabularia (mermaid’s wineglass) is a warm-water alga found in shallow protected lagoons and on the borders of mangrove swamps, growing on shells, coral fragments, and other algae. The thallus is calcified (Kingsley et al., 2003), with less calcification occurring in warm stagnant water. The young
Fig. 5.37 Reconstruction of Ischadites, whole plant and cutaway drawing of the upper portion showing main axis, laterals, and lateral scars. (After Nitecki, 1971.)
single-celled Acetabularia plant has two growing apices, one giving rise to the rhizoidal system that attaches the plants to the substrate, with the other growing apex becoming the erect thallus. As the apex of the axis grows, whorls of sterile hairs are produced just beneath the apex. Each of these whorls is eventually shed, leaving whorls of scars to mark their former attachment positions. During the vegetative growth of the thallus, the nucleus remains in one of the rhizoids.
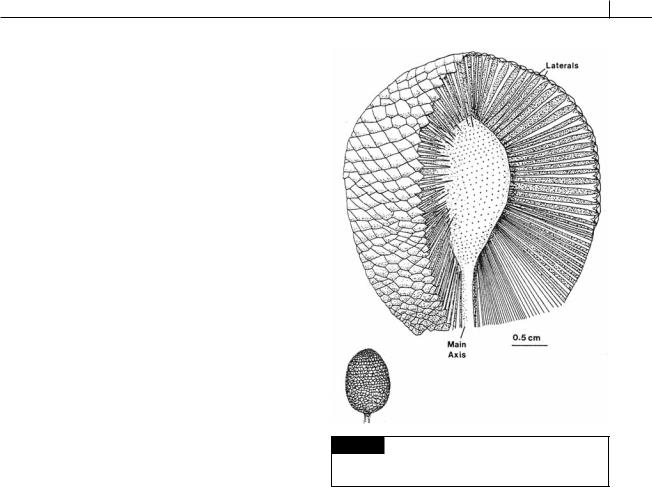
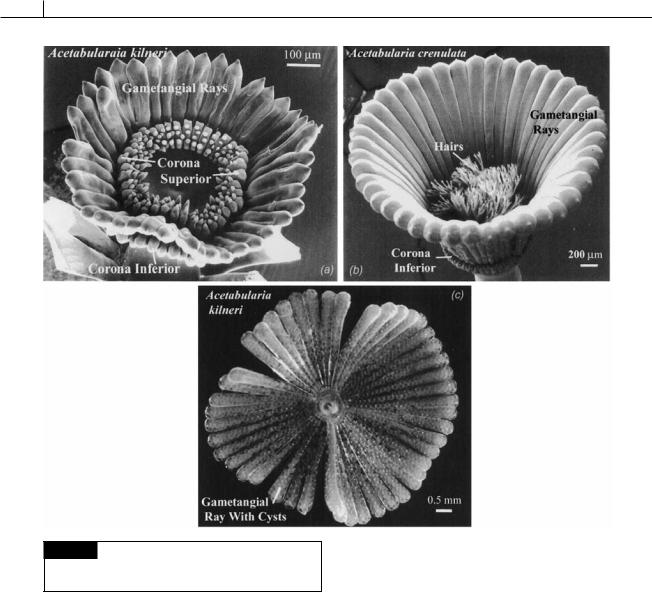
A mature thallus forms a number of gametangial rays at the apex of the thallus, which can be joined or free from each other, depending on the species. Near the base of each gametangial ray, at the tip of the thallus, is a coronal knob, which commonly bears sterile hairs. The coronal knobs together comprise the corona superior. Some species also have a corona inferior beneath the gametangial rays. Once the gametangial rays have reached full size, the primary nucleus in one of
176 EVOLUTION OF THE CHLOROPLAST
Fig. 5.38 Structure of the thallus of Acetabularia. (a) and
(b) are scanning electron micrographs; (c) is a light
micrograph. (From Berger et al., 2003.)
the rhizoids enlarges to about 20 times its original diameter. This nucleus divides by meiosis (Koop, 1975a) into a large number of small secondary nuclei (Woodcock and Miller, 1973a,b), which are carried by cytoplasmic streaming along microtubules into the gametangial rays (Menzel, 1986). In the rays, each nucleus is held in place, a certain distance from other nuclei, by microtubules (Woodcock, 1971). The cytoplasm contracts around each nucleus, and a wall is formed, producing a resistant resting cyst. The cysts enlarge to many times their original size, a process that is accompanied by a number of nuclear divisions. The gametangial rays fall off, with a
plug sealing the supporting part of the thallus (Menzel, 1980). The cysts of Acetabularia mediterranea are usually formed in the summer, are strongly calcified, and do not germinate until the following spring, requiring a resting period of 12 to 15 weeks (Koop, 1975b). At germination of the cyst (Cooper and Mandoli, 1999), the protoplasm divides into a thousand or more pyriform biflagellate isogametes. The gametes are released through a lid in the cell wall. Gametes produced by different cysts are morphologically similar, with gametes from a single cyst probably of the same sex. Parthenogenetic development of gametes has been noted. The zygote can germinate soon after fusing of gametes. In Acetabularia calyculus, gametes are of two sizes. After fusion to form a zygote, the chloroplasts of the smaller, male gametes are preferentially destroyed,
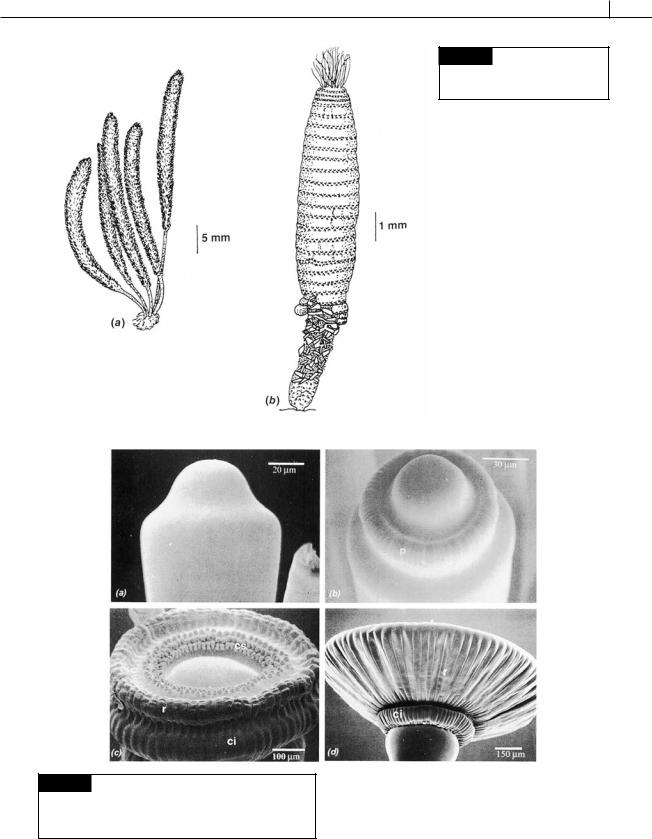
CHLOROPHYTA 177
Fig. 5.39 (a) Dasycladus
vermicularis. (b) Neomeris annulata.
(After Taylor, 1960.)
Fig. 5.40 Scanning electron micrographs of cap initiation in
Acetabularia acetabulum. (ci) Corona inferior; (cs) corona superior; (r) ray cap; (p) primodia. (From Sawitzky et al., 1998.)
