
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
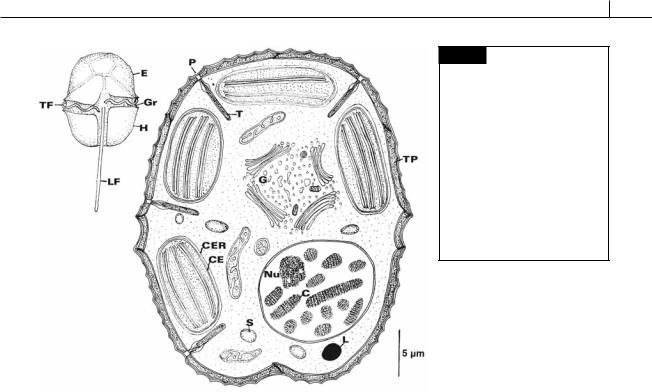
DINOPHYTA 263
Fig. 7.2 Light and electron microscopical drawings of Peridinium sp., a dinoflagellate showing many of the features of the class. (C) Chromosome; (CE) chloroplast envelope; (CER) chloroplast endoplasmic reticulum; (E) epicone;
(G) Golgi apparatus; (Gr) girdle; (H) hypocone; (L) lipid globule; (LF) longitudinal flagellum; (Nu) nucleolus; (P) trichocyst pore; (S) starch; (T) trichocyst; (TF) transverse flagellum; (TP) thecal plate.
membrane of the nuclear envelope. Chlorophylls a and c2 are present in the chloroplasts, with peridinin and neoperidinin being the main carotenoids. About half of the Dinophyceae that have been examined by electron microscopy have pyrenoids in the chloroplasts (Dodge and Crawford, 1970). The storage product is starch, similar to the starch of higher plants (Vogel and Meeuse, 1968), which is found in the cytoplasm. An eyespot may be present. The nucleus has permanently condensed chromosomes and is called a dinokaryotic or mesokaryotic nucleus.
Cell structure
Theca
The thecal structure of motile Dinophyceae consists of an outer plasmalemma beneath which lies a single layer of flattened vesicles (Figs. 7.2, 7.3(c), 7.5) (Dodge and Crawford, 1970; Sekida et al., 2004). These vesicles, which normally contain cellulosic plates, give the theca its characteristic structure. The actual form and arrangement of the thecal plates varies from none in the phagotrophic Oxyrrhis marina, to very thick plates
with flanges at the edges in Ceratium (Figs. 7.12, 7.57) and Peridinium spp. (Figs. 7.2, 7.11).
Amphiesma is the term used to specify the outermost layers of the dinoflagellate cell (Schütt, 1895) and includes all of the thecal plates with their lists (ribs), the peripheral vesicles, and the attendant microtubules. All types of amphiesma have the plasma membranes (continuous with the flagellar membrane) on the outside. The most complex type of amphiesma (Fig. 7.5(a)) consists of a single-membrane-bounded vesicle under the plasma membrane. Inside this vesicle are a number of cellulosic thecal plates subtended by a proteinaceous pellicle (Morrill and Loeblich, 1983a). A less complex amphiesma consists of a number of vesicles under the plasma membrane, each vesicle containing a thecal plate (Fig. 7.5(b), (c)). The least complex amphiesma has a number of vesicles without plates under the plasma membrane (Fig. 7.5(d)). Numerous pores generally occur within the amphiesma with a trichocyst beneath each pore (Fig. 7.4).
Outside of the plasma membrane of Amphidinium carteri (Fig. 7.16) there is a glycocalyx composed of acidic polysaccharides. The glycocalyx probably is formed by the discharge of
264 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
(c)
(f )
|
|
|
|
|
|
(e) |
|
|
(a) |
|
|
|
|
|
|
|
|
|
(b) |
|
|
|
|
|
|
|
|
|
|
(d) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(g) |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
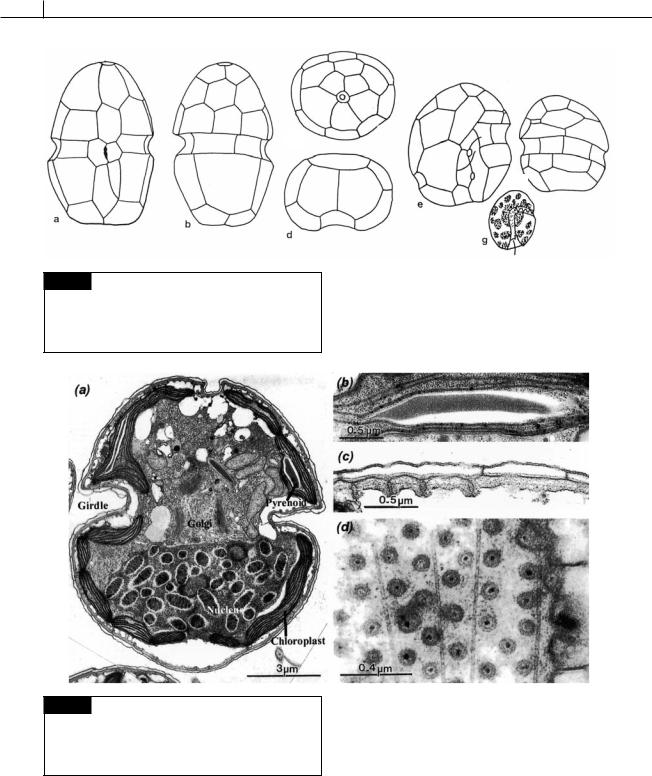
Fig. 7.3 (a)–(d) Cachonina niei showing the arrangement of thecal plates: (a) ventral, (b) dorsal, (c) apical, and (d) posterior views. (e)–(g) Cryptothecodinium cohnii: (e) ventral and (f) dorsal views, (g) living cell. ((a)–(d) after Loeblich, 1968; (e)–(g) after Chatton, 1952.)
Fig. 7.4 Transmission electron micrographs of sections of cells of Karlodinium veneficum. (a) Cell. (b) Detail of pyrenoid in chloroplast. (c) Transverse section of amphiesma showing amphiesmal vesicles. (d) Tangential section of cell showing plugs in amphiesma. (From Daugbjerg et al., 2000.)
cytoplasmic mucocysts to the outside of the cell. Such a glycocalyx probably exists in most Dinophyceae (Klut et al., 1985).
In many dinoflagellates, cell division usually involves sharing of the mother cell thecal plates between the daughter cells, with the daughter cells producing the new thecal plates that they lack. However, in certain peridinoid genera the
DINOPHYTA 265
(a)
(b)
(c)
(d)
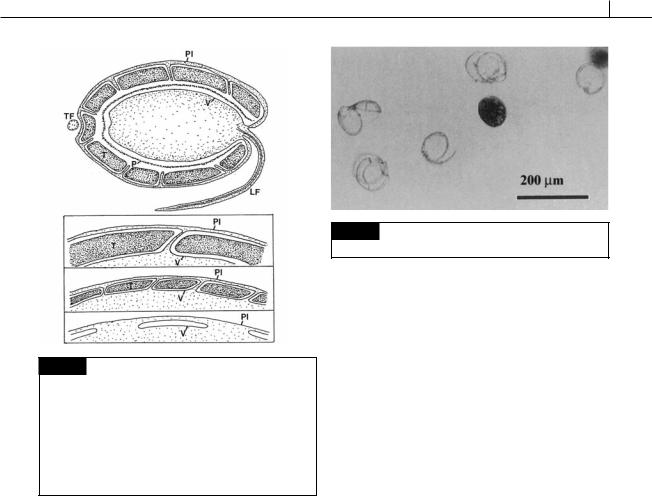
Fig. 7.5 Representative drawings of different types of amphiesmal arrangements in the Dinophyceae. (a) Cross section of a dinoflagellate with the most complex type of amphiesma. The outer plasma membrane (Pl) is continuous with the flagellar membrane. The cellulosic thecal plate (T) and a proteinaceous pellicle (P) are enclosed in a large vesicle
(V). (b)–(d) Less complicated types of amphiesma occurring in other Dinophyceae. (LF) Longitudinal flagellum; (TF) transverse flagellum. (After Morrill and Loeblich, 1983a.)
theca is completely shed (ecdysis) at cell division, followed by the formation of a thickened pellicle around the cell to form an ecdysal cyst (Bricheux et al., 1992; Höhfeld and Melkonian, 1992). New thecal vesicles are produced under the pellicle with the cell escaping from the cyst (Figs. 7.6, 7.7). Ecdysis in Gambierdiscus toxicus is induced by a glycerol compound (Fig. 7.8) that is released by the green alga Bryopsis sp. (Sakamoto et al., 2000). The dinoflagellate occupies the thallisphere of this green alga.
Scales
Although relatively rare, scales occur outside the plasma membrane in some Dinophyceae (Figs. 7.9, 7.10) (Sekida et al., 2003). In Heterocapsa, the scales are formed in Golgi vesicles that migrate to the area of the basal bodies, where the scales are released outside the cell (Morrill and Loeblich, 1983b).
Fig. 7.6 Ecdysis in Gambierdiscus toxicus. (From Sakamoto
et al., 2000.)
Flagella
Generally, dinoflagellates have a transverse flagellum that fits into the transverse girdle and a longitudinal flagellum that projects out from the longitudinal sulcus (Figs. 7.2, 7.11, 7.13) (Roberts and Roberts, 1991). The two flagella are inserted into the cell in the area of the intersection of the girdle and sulcus. The longitudinal flagellum usually has a wide basal portion and a thinner apical portion. In the basal portion the flagellar sheath surrounds packing material plus the axoneme, whereas at the apical portion the flagellar sheath surrounds only the axoneme. Fibrillar hairs 0.5 m long and 10 nm wide may cover the entire flagellum (Leadbeater and Dodge, 1967a). Mechanical stimulus of cells of Ceratium tripos (Fig. 7.12) causes the longitudinal flagellum to be retracted and folded so that the flagellum lies along the sulcus (Maruyama, 1982). The longitudinal flagellum contains an R-fiber running along the length of the flagellum. Retraction of the flagellum occurs when the R-fiber contracts to one third of its length. Influx of Ca2 into the longitudinal flagellum causes retraction of the R-fiber (Maruyama, 1985).
The transverse flagellum is about two to three times as long as the longitudinal flagellum and has a helical shape (Figs. 7.12, 7.13, 7.14). The transverse flagellum consists of (1) an axoneme whose form approximates a helix, (2) a striated strand that runs parallel to the longitudinal axis of the axoneme but outside the loops of the coil, and (3) a flagellar sheath that encloses both axoneme and striated strand (Berdach, 1977). The striated strand
266 CHLOROPLAST E.R.: EVOLUTION OF ONE MEMBRANE
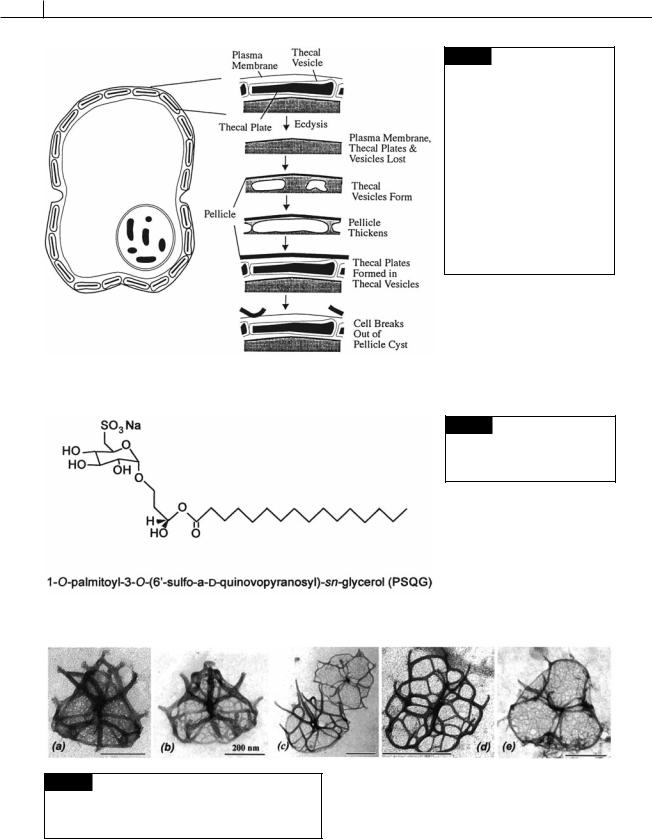
Fig. 7.7 Ecdysis and the formation of new thecal vesicles in Glenodinium foliaceum. Ecdysis results in the loss of the plasma membrane, thecal plates, and vesicles. A pellicle forms from a layer that existed under the thecal vesicles. New thecal vesicles and plates are formed under the thickening pellicle. When the amphiesma is mature, the wall breaks out of the pellicle cyst. (Drawn from transmission electron micrographs in Bricheux et al., 1992.)
Fig. 7.8 The compound released by the green alga Bryopsis that induces ecdysis in the dinoflagellate
Gambierdiscus toxicus.
Fig. 7.9 Transmission electron micrographs of scales of different species of Heterocapsa. (a) H. triquetra. (b) H. arctica.
(c) H. circularisquama. (d) H. horiguchi. (e) H. ildefina. (From Iwataki et al., 2004.)
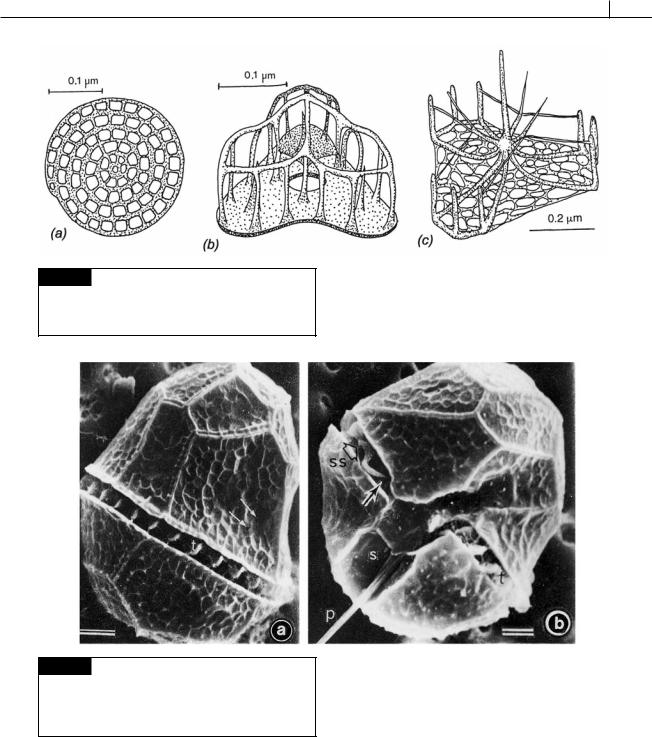
DINOPHYTA 267
Fig. 7.10 Dinoflagellate scales. (a) Oxyrrhis marina. (b)
Heterocapsa niei. (c) Katodinium rotundatum. ((a) after Clarke and Pennick, 1976; (b) after Morrill and Loeblich, 1983b; (c) after Hansen, 1989.)
Fig. 7.11 Dorsal and ventral views of cells of Peridinium cinctum in the scanning electron microscope. (p) Posterior (longitudinal) flagellum; (s) longitudinal sulcus; (ss) striated strand; (t) transverse flagellum. Bar 5 m. (From Berdach, 1977.)
contains centrin, a Ca2 -modulated contractile protein (Höhfeld et al., 1988). Contraction of the striated strand leads to supercoiling of the axoneme. On one side of the axoneme, a unilateral row of fibrillar hairs, 2 to 4 m long and 10 nm wide,
is attached to the flagellar sheath. The final micrometer of the flagellum lacks the striated strand and hairs, with the axoneme tightly covered by the flagellar sheath. The axoneme is a left-handed screw; waves propagated from the attached end toward the free end create a downward thrust at the outermost edges of the coil. The flagellum causes a direct forward movement while at the same time causing the cell to rotate. The flagellar beat always proceeds
