
- •Pineal Parenchymal Tumors
- •Germ Cell Tumors
- •Selected References
- •Medulloblastoma
- •Selected References
- •Anatomy of the Cranial Meninges
- •Meningomas
- •Primary Melanocytic Lesions
- •Other Related Neoplasms
- •Selected References
- •Cranial Nerve Anatomy
- •Schwannomas
- •Neurofibromas
- •Selected References
- •Histiocytic Tumors
- •Selected References
- •Sellar Region Anatomy
- •Normal Imaging Variants
- •Congenital Lesions
- •Neoplasms
- •Miscellaneous Lesions
- •Selected References
- •Intracranial Pseudotumors
- •Selected References
- •Metastatic Lesions
- •Paraneoplastic Syndromes
- •Selected References
- •Scalp Cysts
- •Extraaxial Cysts
- •Parenchymal Cysts
- •Intraventricular Cysts
- •Selected References
- •Anatomy and Physiology of the Basal Ganglia and Thalami
- •Selected References
- •Alcohol and Related Disorders
- •Opioids and Derivatives
- •Inhaled Gases and Toxins
- •Selected References
- •Selected References
- •Hypertensive Encephalopathies
- •Glucose Disorders
- •Thyroid Disorders
- •Seizures and Related Disorders
- •Miscellaneous Disorders
- •Selected References
- •The Normal Aging Brain
- •Dementias
- •Degenerative Disorders
- •Selected References
- •Normal Variants
- •Hydrocephalus
- •CSF Leaks and Sequelae
- •Selected References
- •Cerebral Hemisphere Formation
- •Imaging Approach to Brain Malformations
- •Posterior Fossa Anatomy
- •Chiari Malformations
- •Hindbrain Malformations
- •Selected References
- •Commissural Anomalies
- •Malformations Secondary to Abnormal Postmigrational Development
- •Selected References
- •Anencephaly
- •Holoprosencephaly
- •Holoprosencephaly Variants
- •Related Midline Disorders
- •Holoprosencephaly Mimics
- •Selected References
- •Selected References
- •Selected References
- •Cephaloceles
- •Craniosynostoses
- •Meningeal Anomalies
- •Selected References
- •Index

Congenital Malformations of the Skull and Brain
1294
(40-24) Clinical photograph in a patient with Wyburn-Mason syndrome shows dilated scleral vessels . (Courtesy T. P. Naidich, MD.)
(40-25) Funduscopy in Wyburn-Mason syndrome shows markedly enlarged arteries, veins from retinal AVM. (Courtesy T. P. Naidich, MD.)
Selected References
Capillary Malformation Syndromes
Kirkorian AY et al: Genetic basis for vascular anomalies. Semin Cutan Med Surg. 35(3):128-36, 2016
Sturge-Weber Syndrome
Huang L et al: Somatic GNAQ mutation is enriched in brain endothelial cells in Sturge-Weber syndrome. Pediatr Neurol. 67:59-63, 2017
Pilli VK et al: Clinical and metabolic correlates of cerebral calcifications in SturgeWeber syndrome. Dev Med Child Neurol. ePub, 2017
Pilli VK et al: Enlargement of deep medullary veins during the early clinical course of Sturge-Weber syndrome. Neurology. 88(1):103-105, 2017
Wetzel-Strong SE et al: The pathobiology of vascular malformations: insights from human and model organism genetics. J Pathol. 241(2):281-293, 2017
Comi AM et al: Leveraging a Sturge-Weber gene discovery: an agenda for future research. Pediatr Neurol. 58:12-24, 2016
Kirkorian AY et al: Genetic basis for vascular anomalies. Semin Cutan Med Surg. 35(3):128-36, 2016
Capillary Malformation-Arteriovenous Malformation
Banzic I et al: Parkes Weber syndrome-diagnostic and management paradigms: a systematic review. Phlebology. 32(6):371-383, 2017
Wetzel-Strong SE et al: The pathobiology of vascular malformations: insights from human and model organism genetics. J Pathol. 241(2):281-293, 2017
Other Vascular Phakomatoses
Hereditary Hemorrhagic Telangiectasia
Palagallo GJ et al: The prevalence of malformations of cortical development in a pediatric hereditary hemorrhagic telangiectasia population. AJNR Am J Neuroradiol. 38(2):383-386, 2017
Wetzel-Strong SE et al: The pathobiology of vascular malformations: insights from human and model organism genetics. J Pathol. 241(2):281-293, 2017
Brinjikji W et al: Neurovascular manifestations of hereditary hemorrhagic telangiectasia: a consecutive series of 376 patients during 15 years. AJNR Am J Neuroradiol. 37(8):1479-86, 2016
PHACE Syndrome
Mamlouk MD et al: PHACE syndrome and cerebral cavernous malformations: association or simply microhemorrhages? Childs Nerv Syst. ePub, 2017
Garzon MC et al: PHACE syndrome: consensus-derived diagnosis and care recommendations. J Pediatr. 178:24-33.e2, 2016
Winter PR et al: PHACE syndrome--clinical features, aetiology and management. Acta Paediatr. 105(2):145-53, 2016
Ataxia-Telangiectasia
Vijapura C et al: Genetic syndromes associated with central nervous system tumors. Radiographics. 37(1):258-280, 2017
Blue Rubber Bleb Nevus Syndrome
Soblet J et al: Blue rubber bleb nevus (BRBN) syndrome is caused by somatic TEK (TIE2) mutations. J Invest Dermatol. 137(1):207-216, 2017
Kirkorian AY et al: Genetic basis for vascular anomalies. Semin Cutan Med Surg. 35(3):128-36, 2016
(40-26) DSA in probable Wyburn-Mason syndrome shows a large midbrain AVM . The lesion was treated with stereotaxic radiosurgery.

Chapter 41
1295
Anomalies of the Skull and
Meninges
Anomalies of the skull and meninges represent maldevelopment of the embryonic mesenchyme. These include cephaloceles, other skull base defects such as nasal gliomas or dermoids, congenital calvarial defects, and other meningeal malformations, including lipomas.
We previously discussed how the brain itself develops in Chapter 35. This final chapter of the book focuses on anomalies of the brain coverings, the skull, and meninges. We begin with skull base embryology, which is key to understanding the malformations discussed.
Normal Development and
Anatomy of the Skull Base
Embryology
The skull base (SB) arises primarily from cartilaginous precursors that ossify in an orderly manner from posterior to anterior and from lateral to medial. More than 100 separate ossification centers participate in developing the definitive SB (41-1).
Forehead and Nose
Prior to the eighth week of gestation, two transient but important spaces are present in the developing forehead and nose: the fonticulus frontalis and prenasal space. The fonticulus frontalis lies between the partially ossified frontal bone above and the nasal bones below. The prenasal space is a transient dura-filled structure that lies between the nasal bones and the unossified chondrocranium (41-2A).
As the chondrocranium begins to ossify, it leaves some cartilage in front that later becomes the nasal capsule. By this stage, the fonticulus frontalis has closed. The prenasal space then involutes, leaving a small dural diverticulum anterior to the crista galli called the foramen cecum. The foramen cecum continues anteroinferiorly as a transient dura-lined channel called the anterior neuropore.
The foramen cecum and anterior neuropore establish a temporary connection between the anterior cranial fossa and the nose (41-2B). The anterior neuropore normally regresses completely, leaving a small remnant of the foramen cecum (41-2C). The foramen cecum is approximately 4 mm in diameter at birth and is normally completely ossified by 2 years of age.
Normal Development and |
|
Anatomy of the Skull Base |
1295 |
Embryology |
1295 |
Relevant Gross Anatomy |
1297 |
Cephaloceles |
1298 |
Occipital Cephaloceles |
1299 |
Frontoethmoidal Cephaloceles |
1299 |
Parietal Cephaloceles |
1302 |
Skull Base Cephaloceles |
1302 |
Persistent Craniopharyngeal |
1303 |
Canal |
|
Craniosynostoses |
1305 |
Craniosynostosis Overview |
1305 |
Nonsyndromic Craniosynostosis |
1305 |
Syndromic Craniosynostoses |
1309 |
Meningeal Anomalies |
1311 |
Lipomas |
1311 |
|
|

Congenital Malformations of the Skull and Brain
1296
Skull Base
At birth, the anterior skull base is composed mostly of cartilage with relatively limited ossification. Ossification of the crista galli and cribriform plate begins at 2 months and is nearly complete by 2 years of age. The foramen cecum ossifies last.
The central skull base forms from approximately 24 ossification centers. Major named centers include the presphenoid (planum sphenoidale), postsphenoid (basisphenoid with the posterior half of the sella, dorsum sellae, and upper clivus), alisphenoid (greater sphenoid wing), and orbitosphenoid (lesser sphenoid wing). The intersphenoidal synchondrosis lies between the presphenoid and the postsphenoid (basisphenoid).
The sphenooccipital synchondrosis lies between the basisphenoid and basiocciput. It is one of the last sutures to fuse (not completed until age 15-20 years). The embryonic craniopharyngeal canal—a Rathke pouch remnant—is a
(41-1) Skull base ossification centers show sphenooccipital synchondrosis (between postsphenoid and basiocciput) and craniopharyngeal canal(in intersphenoidal synchondrosis, between preand postsphenoid). (41-2A) Developing dura is white; unossified chondrocranium is blue. Fonticulus frontalis lies between partially ossified frontal and nasal bones. Prenasal space is in dura, between nasal bones/cartilage.
(41-2B) Over time, fonticulus frontalis closes. Chondrocranium is now mostly ossified. Cartilage of developing nasal capsule is blue. Prenasal space, now encased in bone and lined with dura, becomes foramen cecum. Dura-lined channel (anterior neuropore) is open at dorsum of nose. (41-2C) Graphic depicts a later stage of development, by which point anterior neuropore has regressed. Foramen cecum remnant and crista galli are shown.
transient tract from the nasopharynx to the pituitary fossa that passes between chondrification centers for the developing preand postsphenoid bones. The craniopharyngeal canal is typically obliterated by the twelfth gestational week. It is replaced by the transient intersphenoidal synchondrosis, which normally closes around three postnatal months.
The posterior skull base consists primarily of the occipital bone, which has four major ossification centers located around the foramen magnum. In contrast to the anterior and central skull base segments, the posterior skull base is almost completely ossified by birth. However, the sutures remain unfused until the second decade. The petrooccipital and occipitomastoid sutures are among the last of all the cranial sutures to close (15-17 years).
Anomalies of the Skull and Meninges
1297
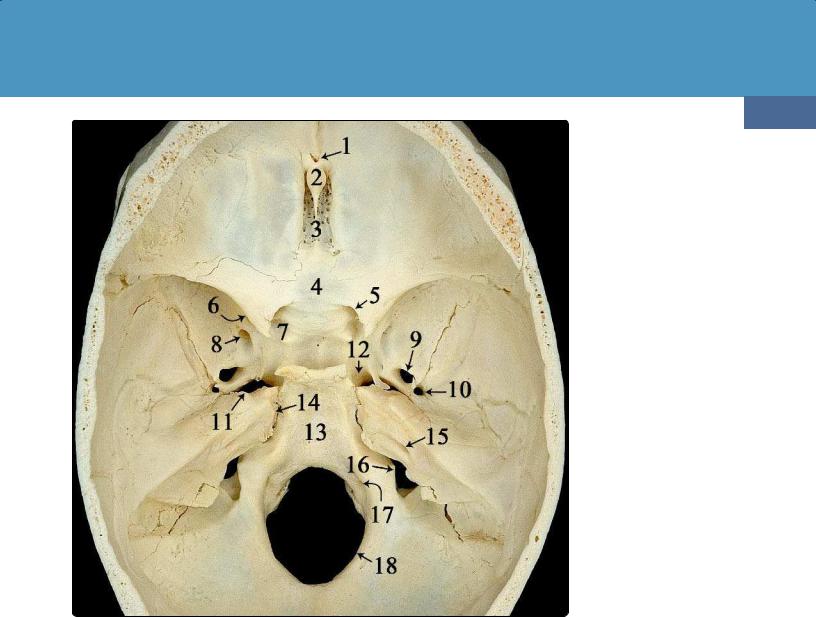
(41-3) Endocranial view of the adult skull shows the following: foramen cecum (1), crista galli (2), cribriform plate (3), planum sphenoidale (4), lesser sphenoid wing and optic canal (5), superior orbital fissure (6), endocranial openings of carotid canal (7, 12), foramen rotundum (8), foramen ovale (9), foramen spinosum (10), foramen lacerum (11), clivus (13), petrooccipital fissure (14), internal auditory canal (15), jugular foramen (16), jugular tubercle overlying hypoglossal canal (17), and the foramen magnum (18). (Courtesy M. Nielsen, MS.)
Relevant Gross Anatomy
Here we briefly delineate the important aspects of the anterior and central segments of the skull base. A detailed description of cranial nerves—their origins, courses, and imaging appearances—is included in Chapter 23.
openings for the internal carotid arteries (7). The optic nerves and ophthalmic arteries pass through the optic canals. The superior orbital fissures transmit the superior orbital veins and oculomotor (CN III) and trochlear (CN IV) nerves, as well as ophthalmic divisions of the trigeminal nerves (CN V ) and the abducens nerves (CN VI).
Anterior Skull Base
The endocranial surface of the anterior skull base (ASB) forms the floor of the anterior cranial fossae. The endocranial surface is composed of the orbital plates of the frontal bones, the ethmoid bone with its cribriform plate and sinus roof, and the lesser sphenoid wing. The exocranial surface of the ASB forms the orbital roofs and abuts the nose.
Important bony landmarks of the endocranial ASB are shown (41-3). In this specimen, the foramen cecum (1) persists as a small bony midline pit immediately in front of the crista galli
(2). The olfactory recesses with the sieve-like cribriform plate
(3) lie on either side of the crista galli. A flat bony surface, the planum sphenoidale (4), extends posteriorly from the cribriform plate of the ethmoid bone to the sella turcica.
The lesser wings of the sphenoid bone overhang the optic canals (5), superior orbital fissures (6), and endocranial
Central Skull Base
The endocranial surface of the central skull base (CSB) forms the sella turcica and medial floors of the middle cranial fossae. It is composed of the greater sphenoid wing, the basisphenoid, and the temporal bone anterior to the petrous ridge. A central depression, the sella turcica, is bordered anteriorly by the tuberculum sellae and anterior clinoid processes. The posterior border of the sella is formed by the dorsum sellae, a prominent bony projection that lies anteromedial to the petrous apices.
Important CSB foramina include the foramen rotundum (8), which transmits the maxillary division of the trigeminal nerve CN V , and the foramen ovale (9), which transmits the mandibular nerve CN V . The foramen spinosum (10) lies posterolateral to the foramen ovale. The middle meningeal artery enters the cranial cavity through the foramen spinosum.
