
- •Contents
- •1. Introduction
- •5. Comments on the Variability of the Diagnoses
- •II. Vienna Consensus Criteria for Pathological Diagnosis
- •1. Vienna Consensus Criteria for Pathological Diagnosis
- •III. Early Neoplasia in Barrett’s Esophagus
- •1. Early Neoplasia in Barrett’s Esophagus
- •1. Gastric Cancer
- •2. Colorectal Cancer
- •3. Esophageal Cancer
- •4. Gastrointestinal Tract Cancer in Europe
- •5. New Trends in Endoscopic Ultrasonography
- •V. Endoscopic Treatment
- •1. Gastric Cancer
- •2. Colorectal Cancer
- •3. Management of Colorectal Cancer by “Hot Biopsy” and Snare Resection
- •4. Esophageal Cancer: Photodynamic Therapy
- •VI. Natural Course of Early Cancer
- •1. Gastric Cancer
- •4. Colorectal Cancer: The Importance of Depressed Lesions in the Development of Colorectal Cancer
- •Index

3. Esophageal Cancer
YOKO MURATA, MASAHIKO OHTA, KAZUHIKO HAYASHI, YOKO HOSHINO, YUKIKO TAKAYAMA, SHINICHI NAKAMURA, and ATSUSHI MITSUNAGA
1. How to Detect Early Esophageal Cancer
1.1 What Is Early Esophageal Cancer?
In 2517 cases of superficial esophageal cancer analyzed in Japan, 287 (11.4%) were intraepithelial cancer, 439 (17.4%) were cancer invading the lamina propria or cancer invading the muscularis mucosae, and 1791
(71.2%) were cancer invading the submucosa. Lymph node metastasis comprised 0% for epithelial cancer, 8.7% for cancer invading the lamina propria or the muscularis mucosae, and 36.5% for cancer invading the submucosa [1]. Following these results, in 1999 the definition of early esophageal cancer changed from cancer invading the submucosa to mucosal cancer without lymph node metastasis according to the Japanese Society of Esophageal Disease [2]. Cancer limited to the submucosa was redefined as superficial cancer [2]. This definition appeared practical, because patients with mucosal cancer had a better survival rate, 96.9% for Tis and 91.9% for cancer invading the lamina propria, compared with 66.9% for patients who had cancer invading the submucosa [3]. Therefore, the early stage of esophageal cancer should be defined as cancer limited to the mucosa.
1.2 Classification of the Depth of
Cancer Invasion
While the number of mucosal cancers is increasing and resected materials have been analyzed, there are different rates of lymph node metastasis and lymphatic invasion according to the depth of invasion [4]. The rate of lymph node metastasis of cancer limited to the epithelium (m1) is 0%, of cancer invading the lamina propria (m2) 3.3%, of cancer reaching the muscularis mucosae (m3) 12%, of cancer slightly invading the submucosa (sm1) 14%, of cancer invading further than the middle of the submucosa (sm2) 35.8%, and of cancer reaching the proper muscle (sm3) 45.9% [4]. The rates of lymphatic invasion and blood vessel invasion of m1 were 1.0% and 0.3%, of m2 6.5% and 0.4%, of m3 23.1% and 4.3%, of sm1 40.7% and 12.9%, and of sm2,3 52.8%, 67.3% and 22.2%, 32.9%, respectively [4]. Thus, m1 and
m2 had a low rate of lymph node metastasis and lymphatic and vessel invasion, so that local treatments such as mucosal resection via endoscopy could be introduced; m3 and sm1 had a low rate of lymph node metastasis but a high rate of lymphatic invasion, so that cases without lymph node swelling could be selected for local treatments, and after lymphatic invasion was recognized in the resected specimen chemotherapy could be performed; while sm2,3 had a high rate of lymph node metastasis so that surgical operation with lymph node dissection could be recommended.
1.3 Symptoms of Early Esophageal
Cancer: Detection of Early Esophageal
Cancer in Japan
Clinically, in 500 cases of mucosal cancer 56.4% of patients with superficial cancer were asymptomatic, 6.8% of patients had retrosternal pain, and 9% had feelings of stenosis, some involving foreign body sensation, dysphagia, nausea, and so on [3]. Sixty-eight percent of patients had no symptoms or unrelated complaints, and only 37% of cancers were detected based on symptoms [3]. Nabeya reported that 100% of Tis cancers and 44% of mucosal cancers were detected by mass screening or physical checkup [1] and 62% of cancers invading the submucosa were detected based on symptoms. Kodama and Kakegawa also reported that 55% of patients with superficial cancer were asymptomatic [4]. Regarding the detection method, 91% of Tis cancers and 64% of mucosal cancers were detected by endoscopy, but 76% of cancers invading the submucosa were detected by upper gastrointestinal tract X-ray. Koyama reported that m1–m2 cancer that has already been diagnosed by endoscopy can be detected by esophagography in 27% of cases by inexperienced doctors and in 68% of cases by experienced doctors [5]. Most cases of mucosal cancer were asymptomatic and were detected incidentally by endoscopy at the second stage of mass screening or physical checkup. (People are persuaded to have an endoscopic examination when abnormal lesions in the stomach are detected by X-ray examination at the
first stage of mass screening.) On the other hand, most cancers invading the submucosa could be detected by X-ray examination.
171

172 IV. Detection of Early Cancer: Is Endoscopic Ultrasonography Effective
1.4 Which Active Methods Can Detect |
1.5 Which Endoscopic Findings are |
Early Esophageal Cancer? |
Indicative of Early Esophageal Cancer? |
Methods of early esophageal cancer detection in a highrisk group, involving factors such as being more than 55 years of age, of male sex, having a habit of smoking and drinking, having head and neck cancer (esophageal cancer found in 11.8% of patients) [6], achalasia, lye esophagitis, and Barrett’s esophagus have been recommended. Endoscopic examination followed by iodine staining has been performed for patients in a high-risk group. Endoscopy followed by iodine staining has a major role in the diagnosis of mucosal cancer. Miyaji et al. reported that esophageal cancer was found in 12.7% of patients with head and neck cancer [7]. Therefore, patients in a high-risk group should undergo endoscopic examination including iodine staining during mass screening.
First of all, careful examination of the esophagus is necessary when the endoscope is inserted into and withdrawn from the esophagus; the esophageal wall should not be extended too much, while close observation during esophageal movement is also important. Extremely small abnormalities, such as change of color, redness (Fig. 1), abnormal vessels, translucent area, whitish area (Fig. 2), slightly depressed (Fig. 3) and elevated lesions (Fig. 2), granular or rough surface, should be checked and iodine staining performed. For iodine staining, 1.5%–2% iodine solution should be sprayed into the whole esophagus. Esophageal cancer, dysplasia, esophagitis, acanthosis, hyperkeratosis, ulcer, erosion, gastric mucosa, and columnar epithelium lacking glycogen, are not stained by iodine. The size of the unstained
Fig. 1. Type 0–IIc+IIa. A slight depression with redness and slightly whitish elevated lesion is not stained by iodine
Fig. 2. Type 0–IIa. A slightly elevated and whitish lesion is not stained by iodine

Fig. 3. Type 0–IIc. A slight depression with flat surface is not stained by iodine. There are two lesions in this case
Fig. 4. Type 0–I. A nodular protruding tumor is not stained by iodine
area is important: an unstained area of less than 5 mm has a lower probability of cancer (10%). On the other hand, a 6–10-mm unstained area has a 19% chance of cancer and an unstained area of more than 10 mm has a more than 55% chance of cancer [8]. As a consequence, any unstained area of more than 5 mm should be biopsied.
1.6 Determination of the Depth of Cancer Invasion by Endoscopy
It is helpful to classify the macroscopic type of a lesion, such as protruding type (0–I) (Fig. 4), slightly elevated (0–IIa) (Fig. 2), flat (0–IIb) (Fig. 5), slightly depressed (0–IIc) (Fig. 3), and excavated type (0–III), to distin-
3. Esophageal Cancer |
173 |
guish mucosal cancer from cancer invading the submucosa. Yoshida et al. reported that 92% of cancers of type 0–I comprised cancer invading the submucosa, 96% of type 0–III also involved cancer invading the submucosa, and 85% of type 0–II was limited to the mucosa, in a cohort of 350 cases of superficial cancer [9]. These authors reported that 15% of type 0–IIa and 20% of type 0–IIc were cancers invading the submucosa, but all of type 0–IIb were mucosal cancers. Therefore, lesions with protruded or excavated factors relate to cancer invading the submucosa, while superficial lesions associated with color changes tend to be mucosal cancer. Among types 0–IIa and 0–IIc, 15%–20% invade the submucosa. Regarding 0–IIa lesions, diagnostic points indicative of mucosal cancer are a height of the elevated elements of less than 2 mm, a shape that is

174 IV. Detection of Early Cancer: Is Endoscopic Ultrasonography Effective
granular but not nodular, and a whitish color of the lesion. In 0–IIc lesions, the abscence of a wall and a flat (Fig. 3) or granular (Fig. 6) surface with no nodule are findings suggestive of cancer limited to the mucosa. If lesions have mixed elements, such as IIa+IIc or IIa+large IIb, cancer invading the submucosa is suspected [4]. If the lesion is 0–IIb or whitish 0–IIa, intraepithelial cancer (m1) is suspected.
2. Endoscopic Ultrasonography
(EUS) in Early Esophageal Cancer
2.1 What Is the Role of EUS? How to Use EUS
The role of endoscopic ultrasonography (EUS) is not only to determine the depth of cancer invasion but also to detect lymph nodes. Several ultrasonic probes have been developed. Catheter-type probes (30 or 20 MHz, 2.6–2.4 mm in diameter) have been introduced for the diagnosis of superficial esophageal invasion. Conven-
Fig. 5. Type 0–IIb. A slightly red, flat area is not stained by iodine
Fig. 6. Type 0–IIc. A slight depression with granular surface is not stained by iodine
tional probes (7.5 MHz, 12 MHz) are used for detecting lymph nodes in the posterior mediastium and the abdomen. The method of determining the depth of cancer invasion involves the patient lying on his or her left side. The two-channel scope is inserted near the lesion, the lesion is observed, and the catheter-type probe is inserted through the channel while a tube of irrigating water is passed through the other channel. The scanning is started when the lesion is submerged in water.
2.2 Normal Esophageal Wall Structure
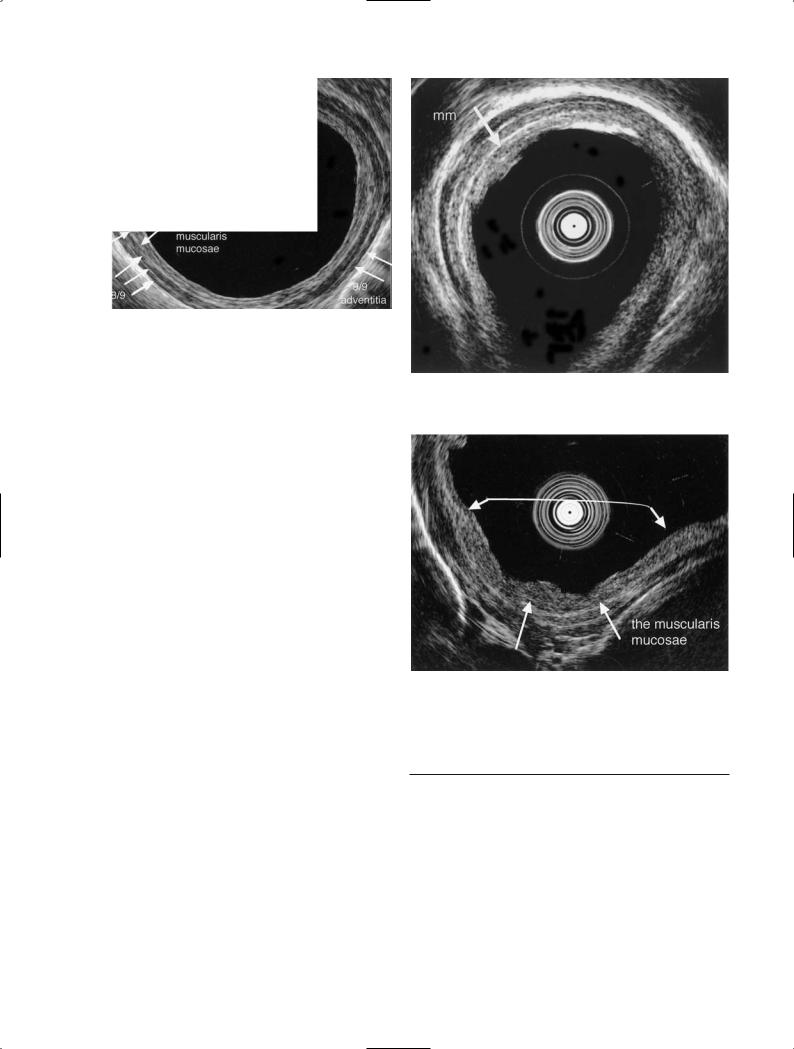
The normal esophageal wall is delineated as a structure of nine layers: the 1st and 2nd layer (1, 2/9) comprise the epithelium, and the 3rd (3/9) is the lamina propria.
The 4th layer (4/9), the hypoechoic layer, is the muscularis mucosae which is important in distinguishing between cancer involving the lamina propria and cancer invading deeper than the muscularis mucosae. The 5th layer (5/9), the hyperechoic layer, is the submucosa, the
3. Esophageal Cancer |
175 |
Fig. 7. Normal esophageal wall structure. The 1st and 2nd layers (1,2/9) are the epithelium, the 3rd (3/9) is the lamina propria, the 4th (4/9) is the muscularis mucosae, the 5th (5/9) is the submucosa, from the 6th (6/9) to the 8th (8/9) are the muscularis propria, and the 9th (9/9) is the adventitia
6th (6/9) to the 8th (8/9) are the muscularis propria, and the 9th (9/9) is the adventitia (Fig. 7) [10].
Fig. 8. Cancer limited to the epithelium (m1): Tis. The tumor is limited to the 1st (1/9) and the 2nd (2/9) layers (arrow)
2.3 Determination of the Depth of Cancer Invasion: Extent of Accuracy
The depth of cancer invasion can be determined by observing which layers are destroyed or remain normal.
m1: The tumor is limited to the 1st (1/9) and the 2nd (2/9) layers (Fig. 8).
m2: The tumor invades a part of the 3rd layer (3/9); however, the 4th layer (4/9) is preserved under the tumor (Fig. 9).
T1-m3–sm1: The tumor destroys the 4th layer, but the 5th layer (5/9) is preserved under the tumor.
T1-sm2,3: The tumor destroys a part of or whole of the 5th layer, and the 6th layer (6/9) is preserved.
In 113 patients with superficial esophageal cancer, EUS was performed and histological findings were compared with EUS findings. Regarding determination of the depth of cancer invasion, accuracy for m1 was 38%, for m2 95%, for m3 and sm1 62%, and for sm2,3 86%. The overall accuracy was 75%, and the accuracy for determining invasion of less than m2 was 95% (Table 1). Fukuda et al. reported that the accuracy of distinguishing m and sm was 85% [11]. m1 was overestimated as m2 because lymph follicles or lymphocyte infiltration in the lamina propria, which are also observed as hypoechoic areas, made it difficult to diagnose cancer invasion correctly. After the introduction of 30 MHz EUS the muscularis mucosae could be clearly delineated, resulting in an increased accuracy.
Fig. 9. Cancer invading the lamina propria (m2). The tumor is invading a part of the 3rd layer (3/9); however, the 4th layer (4/9) is preserved under the tumor
Table 1. Relationship between endoscopic ultrasonography (EUS) findings and histologic results in esophageal cancer
EUS findings |
|
|
Histology |
|
|
Tis |
T1lpm |
T1mm–sm1 |
T1sm2,3 |
Tis |
5 |
1 |
|
|
T1lpm |
8 |
12 |
|
1 |
T1 mm–sm1 |
|
1 |
8 |
1 |
T1sm2,3 |
|
3 |
5 |
60 |
T2 |
|
|
|
8 |
|
5/13 |
12/17 |
8/13 |
60/70 |
|
38% |
71% |
62% |
86% |
Overall accuracy |
|
|
|
85/113 (75%) |
Determination of cancer limited to the lamina |
|
|||
propria: |
|
|
|
108/113 (95%) |
|
|
|
|
|

176 IV. Detection of Early Cancer: Is Endoscopic Ultrasonography Effective
2.4 Determination of Lymph
Node Metastasis
Lymph nodes more than 3 mm in diameter located in the posterior mediastium, around the celiac axis, and near the stomach can be delineated by conventional EUS (12 or 7.5 MHz). Endoscopic ultrasonography findings of metastasis were compared with histological results in patients who had undergone a thoracotomy. The sensitivity was 90%, the specificity was 51%, and the overall accuracy was 75%. Since EUS fine-needle aspiration cytology (EUS-FNA) has been performed safely and the accuracy of EUS-FNA for lymph node metastasis has been reported to be around 83%–96% [12], suspected positive lymph nodes in mucosal cancer should be confirmed using EUS-FNA.
References
1.Nabeya K (1993) Early carcinoma of the esophagus. In: Nabeya K, Hanaoka T, Nogami H (eds) Recent advances in diseases of the esophagus Springer, Berlin Heidelbery New York Tokyo, 1993, pp 375–380
2.Japanese Society for Esophageal Diseases (1999) Guide lines for the clinical and pathologic studies on carcinoma of the esophagus. 9th edn. Kanehara, Tokyo
3.Endo M, Yoshino K, Kawano T, et al (1992) Clinical evaluation of mucosal cancer of the esophagus: analysis of 1583 cases of superficial esophageal resected in Japan. In: Nabeya, et al (eds) Diseases of the esophagus. SpringerVerlag, Tokyo, pp 540–545
4.Kodama M, Kakegawa T (1998) Treatment of superficial cancer of esophagus: a summary of responses to questionnaire on superficial cancer of the esophagus in Japan. Surgery 123:432–439
5.Oyama T, Hotta K, Shimaya S, et al (2001) Is esophagography useful for the diagnosis of superficial esophageal cancer? Endosc Dig 13:29–32
6.Makuuchi H, Machimura T, Shimada H, et al (1996) Endoscopic screening for esophageal cancer in 788 patients with head and neck cancers. Tokai Exp Clin Med 21:139–145
7.Miyaji M, Makuuchi H, et al (eds) (1997) Handbook for early esophageal cancer. Chugai Medical, Tokyo, pp 48–47
8.Ide H, Itabashi M (1991) Application of the staining technique in resected specimens: its contribution to the diagnosis of mucosal cancer and slight pathological changes of the mucosa. In: Endo M, Ide H (eds) Endoscopic staining in early diagnosis of esophageal cancer. Japan Scientific Societies Press, Tokyo, pp 47–65
9.Yoshida M, Momma K, Hanashi T, et al (2001) Endoscopic evaluation of depth of cancer invasion in cancer with superficial esophageal cancer. Stomach Intest 36: 295–306
10.Murata Y, Suzuki S, Ohta M, et al (1996) Small ultrasonic probes for determination of the depth of superficial esophageal cancer. Gastrointest Endosc 44:23–28
11.Fukuda M, Hirata K, Natori H (2000) Endoscopic ultrasonography of the esophagus. World J Surg 24: 216–218
12.Barawai M, Gress F (2000) EUS-guided fine-needle aspiration in the mediastinum. Gastrointestinal Endosc 52(Suppl 6):12–17
