
Книги по МРТ КТ на английском языке / Advanced Imaging of the Abdomen - Jovitas Skucas
.pdf
104
ADVANCED IMAGING OF THE ABDOMEN
plication assessment. Gastroenterology 1997;112: 1087–1095.
5.Ng CC, Hung FC, Hsieh CS, et al. Epidermolysis bullosa letalis with pyloric atresia in an infant. J Formos Med Assoc 1996;95:61–65.
6.Guadagni S, Gola P, Marsili L, et al. Arterial vasculature of the stomach and oncologic gastrectomies. [Review] Surg Radiol Anat 1995;17:269–276.
7.Hsu PI, Lai KH, Tseng HH, et al. Impact of Helicobacter pylori eradication on the development of MALT, gland atrophy and intestinal metaplasia of the antrum. Chin Med J 2000;63:279–287.
8.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sidney system. Am J Surg Pathol 1996;20:1161–1181.
9.Bender GN, Peller T, Tsuchida A, Kelley JL. Double contrast upper gastrointestinal barium examination with biopsy versus endoscopy with biopsy in dyspeptic patients. Invest Radiol 1995;30:329–333.
10.Loffeld RJ. Diagnostic value of endoscopic signs of gastritis: with special emphasis to nodular antritis. Neth J Med 1999;54:96–100.
11.Chapman PR, Boals JR. Pneumopericardium caused by giant gastric ulcer. AJR 1998;171:1669–1670.
12.D’Inca R, Sturniolo G, Cassaro M, et al. Prevalence of upper gastrointestinal lesions and Helicobacter pylori infection in Crohn’s disease. Dig Dis Sci 1998;43: 988–992.
13.Sanguino JC, Rodrigues B, Baptista A, Quina M. Focal lesion of African histoplasmosis presenting as a malignant gastric ulcer. Hepatogastroenterology 1996;43: 771–775.
14.Radhi J, Kamouna M, Nyssen J. Phlegmonous gastritis following coronary bypass surgery. Can J Gastroenterol 1999;13:837–839.
15.Millward SF, Fortier M. Transient gastric emphysema caused by colonic infarction. AJR 2001;176:1331–1332.
16.Greaney TV, Nolan N, Malone DE. Multiple gastric polyps in familial amyloid polyneuropathy. Abdom Imaging 1999;24:220–222.
17.Joko T, Tanaka H, Hirakata H, et al. Phlegmonous gastritis in a haemodialysis patient with secondary amyloidosis. Nephrol Dial Transplant 1999;14:196–198.
18.Papa A, Cammarota G, Tursi A, et al. Histologic types and surveillance of gastric polyps: a seven year clinicopathological study. Hepatogastroenterology 1998;45: 579–582.
19.Cherukuri R, Levine MS, Furth EE, Rubesin SE, Laufer I. Giant hyperplastic polyps in the stomach: radiographic findings in seven patients. AJR 2000;175: 1445–1448.
20.Matsushita M, Hajiro K, Okazaki K, Takakuwa H. Gastric inflammatory fibroid polyps: endoscopic ultrasonographic analysis in comparison with the histology. Gastrointest Endosc 1997;46:53–57.
21.Cho JS, Shin KS, Kwon ST, et al. Heterotopic pancreas in the stomach: CT findings. Radiology 2000;217: 139–144.
22.Park SH, Han JK, Choi BI, et al. Heterotopic pancreas of the stomach: CT findings correlated with pathologic findings in six patients. Abdom Imaging 2000;25: 119–123.
23.Palmer GM, Shortsleeve MJ. Gastric polyps due to extramedullary hematopoiesis. AJR 1998;171:531.
24.Kim SH, Han JK, Yoon CJ, et al. Gastric adenoma with atypical appearance: findings on double-contrast barium study with histopathologic correlation.Abdom Imaging 2000;25:124–128.
25.Ferrozzi F,Tognini G,Zuccoli G,Gabrielli M,Zangrandi A, Campani R. [Gastric stromal tumors. Findings with computerized tomography.] [Italian] Radiol Med 2000; 99:56–61.
26.Thompson WM, Kende AI, Levy AD. Imaging characteristics of gastric lipomas in 16 adult and pediatric patients. AJR 2003;181:981–985.
27.Galant J, Marti-Bonmati L, Saez F, Soler R, AlcalaSantaella R, Navarro M. The value of fat-suppressed T2 or STIR sequences in distinguishing lipoma from well-differentiated liposarcoma. Eur Radiol 2003;13: 337–343.
28.Theuer CP, Kurosaki T, Taylor TH, Anton-Culver H. Unique features of gastric carcinoma in the young: a population-based analysis. Cancer 1998;83:25–33.
29.Dorant E, van den Brandt PA, Goldbohm RA, Sturmans F. Consumption of onions and a reduced risk of stomach carcinoma. Gastroenterology 1996;110:12–20.
30.Adachi Y, Mori M, Maehara Y, Matsumata T, Okudaira Y, Sugimachi K. Surgical results of perforated gastric carcinoma: an analysis of 155 Japanese patients. Am J Gastroenterol 1997;92:516–518.
31.Arikawa J, Tokunaga M, Satoh E, Tanaka S, Land CE. Morphological characteristics of Epstein-Barr virusrelated early gastric carcinoma: a case-control study. Pathol Int 1997;47:360–367.
32.Kaneko S, Yoshimura T. Time trend analysis of gastric cancer incidence in Japan by histological types, 1975–1989. Br J Cancer 2001;84:400–405.
33.Park M-S, Ha HK, Choi BS, et al. Scirrhous Gastric Carcinoma: Endoscopy versus Upper Gastrointestinal Radiography. Radiology 2004;231:421–426.
34.Shindoh N, Nakagawa T,Ozaki Y, Kyogoku S, Sumi Y, Katayama H. Overlooked gastric carcinoma: pitfalls in upper gastrointestinal radiology. Radiology 2000;217: 409–414.
35.Takao M, Fukuda T, Iwanaga S, Hayashi K, Kusano H, Okudaira S. Gastric cancer: evaluation of triphasic spiral CT and radiologic-pathologic correlation. J Comput Assist Tomogr 1998;22:288–294.
36.Kim HS, Han HY, Choi JA, et al. Preoperative evaluation of gastric cancer: value of spiral CT during gastric arteriography (CTGA). Abdom Imaging 2001;26:123–130.
37.Lee DH, Ko YT. The role of 3D spiral CT in early gastric carcinoma. J Comput Assist Tomogr 1998;22:709–713.
38.Lee JH, Jeong YK, Kim DH, et al. Two-phase helical CT for detection of early gastric carcinoma: importance of the mucosal phase for analysis of the abnormal mucosal layer. J Comput Assist Tomogr 2000;24:777– 782.
39.Monig SP, Zirbes TK, Schroder W, et al. Staging of gastric cancer: correlation of lymph node size and metastatic infiltration. AJR 1999;173:365–367.
40.Dux M, Richter GM, Hansmann J, Kuntz C, Kauffmann GW. Helical hydro-CT for diagnosis and staging of gastric carcinoma. J Comput Assist Tomogr 1999;23: 913–922.
41.Rossi M, Broglia L, Graziano P, et al. Local invasion of gastric cancer: CT findings and pathologic correlation

105
STOMACH
using 5–mm incremental scanning, hypotonia, and water filling. AJR 1999;172:383–388.
42.Lim HK, Kim S, Lim JH, et al. Assessment of pancreatic invasion in patients with advanced gastric carcinoma: usefulness of the sliding sign on sonograms. AJR 1999;172:615–618.
43.Kim AY, Han JK, Kim TK, Park SJ, Choi BI. MR imaging of advanced gastric cancer: comparison of various MR pulse sequences using water and gadopentetate dimeglumine as oral contrast agents. Abdom Imaging 2000;25:7–13.
44.Sohn KM, Lee JM, Lee SY, Ahn BY, Park SM, Kim KM. Comparing MR imaging and CT in the staging of gastric carcinoma. AJR 2000;174(6):1551–1557.
45.Kang BC, Kim JH, Kim KW, et al. Value of the dynamic and delayed MR sequence with Gd-DTPA in the T-staging of stomach cancer: correlation with the histopathology. Abdom Imaging 2000;25:14– 24.
46.Marrelli D,Roviello F,De Stefano A,et al. Surgical treatment of gastrointestinal carcinomas in octogenarians: risk factors for complications and long-term outcome. Eur J Surg Oncol 2000;26:371–376.
47.Park KB, Do YS, Kang WK, et al. Malignant obstruction of gastric outlet and duodenum: palliation with flexible covered metallic stents. Radiology 2001;219: 679–683.
48.Jung GS, Song HY, Kang SG, et al. Malignant gastroduodenal obstructions: treatment by means of a covered expandable metallic stent-initial experience. Radiology 2000;216:758–763.
49.Lee SS, Ha HK, Kim AY, et al. Primary extrapulmonary small cell carcinoma involving the stomach or duodenum or both: findings on CT and barium studies. AJR 2003;180:1325–1329.
50.Shikuwa S, Senju M, Tanaka H, et al. Progressive systemic sclerosis associated with primary small cell carcinoma of the stomach. [Review] J Gastroenterol 1997;32:538–542.
51.Hansen PB, Vogt KC, Skov RL, Pedersen-Bjergaard U, Jacobsen M, Ralfkiaer E. Primary gastrointestinal nonHodgkin’s lymphoma in adults: a population-based clinical and histopathologic study. J Intern Med 1998; 244:71–78.
52.Matsumoto S, Kohda K, Koike K, et al. [Diagnosis and prognosis of reactive lymphoreticular hyperplasia (RLH) or mucosa-associated lymphoid tissue (MALT) lymphoma.] [Japanese] Nippon Shokakibyo Gakkai Zasshi 1998;95:872–879.
53.Andoh A, Takaya H, Bamba M, et al. Primary gastric Burkitt’s lymphoma presenting with c-myc gene rearrangement. J Clin Gastroenterol 1998;33:710– 715.
54.Kinoshita K, Hirota S, Isozaki K, et al. Absence of c-kit gene mutations in gastrointestinal stromal tumours from neurofibromatosis type 1 patients. J Pathol 2004; 202:80–85.
55.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708–710.
56.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics 2003;23:283–304.
57.Ghanem N, Altehoefer C, Furtwangler A, et al. Computed tomography in gastrointestinal stromal tumors. Eur Radiol 2003;13:1669–1678.
58.Chak A, Canto MI, Rosch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc 1997;45:468–473.
59.Rha SE, Sohn KM, Lee SY, Jung HS, Park SM, Kim KM. Pedunculated exogastric leiomyoblastoma presenting as a wandering abdominal mass. Abdom Imaging 2000;25:545–547.
60.Sofka CM, Semelka RC, Marcos HB, Calvo BF, Woosley JT. Metastatic gastric leiomyoblastoma: a case report. Magn Reson Imaging 1998;16:343–346.
61.Seki K, Hasegawa T, Konegawa R, Hizawa K, Sano T. Primary liposarcoma of the stomach: a case report and a review of the literature. [Review] Jpn J Clin Oncol 1998;28:284–288.
62.Usuda H, Naito M. Multicentric angiosarcoma of the gastrointestinal tract. [Review] Pathol Int 1997;47:553– 556.
63.Kim JK, Won JH, Cho YK, Kim MW, Joo HJ, Suh JH. Glomus tumor of the stomach: CT findings. Abdom Imaging 2001;26:303–305.
64.Tsai SC, Hsieh JF, Ho YJ, Kao CH. Effects of butter and soybean oils on solid-phase gastric emptying in patients with functional dyspepsia. Abdom Imaging 2000;25:35–37.
65.Lauenstein TC, Vogt FM, Herborn CU, DeGreiff A, Debatin JF, Holtmann G. Time-resolved threedimensional MR imaging of gastric emptying modified by IV administration of erythromycin. AJR 2003;180:1305–1310.
66.Halkar RK, Paszkowski AL, Jones ME, et al. Two-point, timesaving method for measurement of gastric emptying with diagnostic accuracy comparable to that of the conventional method. Radiology 1999;213:599– 602
67.Trioche P, Chalas J, Francoual J, et al. Jaundice with hypertrophic pyloric stenosis as an early manifestation of Gilbert syndrome. Arch Dis Child 1999;81:301–303.
68.Hernanz-Schulman M, Lowe LH, Johnson J, et al. In vivo visualization of pyloric mucosal hypertrophy in infants with hypertrophic pyloric stenosis: is there an etiologic role? AJR 2001;177:843–848.
69.Yamamoto A, Kino M, Sasaki T, Kobayashi Y. Ultrasonographic follow-up of the healing process of medically treated hypertrophic pyloric stenosis. Pediatr Radiol 1998;28:177–178.
70.Veyrac C, Couture A, Bongrand AF, Baud C, Ferran JL. Atypical pyloric stenosis in an infant with familial hyperlipidemia. Pediatr Radiol 1996;26:402–404.
71.Graadt van Roggen JF, van Krieken JH. Adult hypertrophic pyloric stenosis: case report and review. [Review] J Clin Pathol 1998;51:479–480.
72.Skarsgard PL, Blair GK, Culham G. Balloon pyloroplasty in children with delayed gastric emptying. Clin Radiol 1998;41:151–155.
73.Kao CH, ChangLai SP, Chieng PU,Yen TC. Gastric emptying in male neurologic trauma. J Nucl Med 1998; 39:1798–1801.
74.O’Hara SM, Donnelly LF, Chuang E, Briner WH, Bisset GS 3rd. Gastric retention of zinc-based pennies: radiographic appearance and hazards. Radiology 1999;213: 113–117.

106
ADVANCED IMAGING OF THE ABDOMEN
75.McCaig J, Varghese JC, Rees MR. Case report: transdiaphragmatic gastric herniation: a rare complication of CABG using the right gastroepiploic artery. Clin Radiol 1996;51:143–145.
76.Privat C, Courthaliac C, Ravel A, Perez N, Boiteux JP, Boyer L. [CT and MRI features of a pseudo adrenal lesion: subcardial diverticulum.] [French] Prog Urol 1999;9:509–512.
77.Fabian G, Tovari E, Baranyay F, Czirjak L. Watermelonstomach as a cause of chronic iron deficiency anemia in a patient with systemic sclerosis. J Eur Acad Dermatol Venereol 1999;12:161–164.
78.Misra V, Misra SP, Dwivedi M. Thickened gastric mucosal capillary wall: a histological marker for portal hypertension. Pathology 1998;30:10–13.
79.Chang D, Levine MS, Ginsberg GG, Rubesin SE, Laufer I. Portal hypertensive gastropathy: radiographic findings in eight patients. AJR 2000;175:1609–1612.
80.Carucci LR, Levine MS, Rubesin SE, Laufer I. Tumorous gastric varices: radiographic findings in 10 patients. Radiology 1999;212:861–865.
81.Ninoi T, Nakamura K, Kaminou T, et al. TIPS versus transcatheter sclerotherapy for gastric varices. AJR 2004;183:369–376
82.Chau TN, Patch D, Chan YW, Nagral A, Dick R, Burroughs AK. “Salvage” transjugular intrahepatic portosystemic shunts: gastric fundal compared with esophageal variceal bleeding. Gastroenterology 1998; 114:981–987.
83.Hirota S, Matsumoto S, Tomita M, Sako M, Kono M. Retrograde transvenous obliteration of gastric varices. Radiology 1999;211:349–356.
84.Contractor QQ, Contractor TQ, Kher YR. Sclerotherapy in bleeding gastric varices of hepatic schistosomiasis. J Clin Gastroenterol 1996;23:97–100.
85.Collazos J, Blanco MS, Mayo J, Martinez E. Gastrointestinal hemorrhage due to gastroduodenal involvement by Mycobacterium avium complex in a patient with acquired immune deficiency syndrome. J Clin Gastroenterol 1998;26:84–85.
86.Alcantara Gijon F, Bernal Bellido C, Ceballos Garcia R,
Gomez Munoz JC. [Gastric necrosis due to vasculitis in an HIV+ patient.] [Review] [Spanish] Rev Esp Enferm Dig 1999;91:152–153.
87.Mikala G, Xie J, Berencsi G, et al. Human herpesvirus 8 in hematologic diseases. [Review] Pathol Oncol Res 1999;5:73–79.
88.Zoller WG, Bogner JR, Powitz F, Liess H, Goebel FD. Endoscopic ultrasound in the diagnosis and staging of gastrointestinal Kaposi’s sarcoma. Endoscopy 1995;27: 191–196.
89.Carbognin G, Biasiutti C, El-Khaldi M, Ceratti S, Procacci C. Afferent loop syndrome presenting as enterolith after Billroth II subtotal gastrectomy: a case report. Abdom Imaging 2000;25:129–131.
90.Yu J, Turner MA, Cho S-R, et al. Normal anatomy and complications after gastric bypass surgery: Helical CT findings. Radiology 2004;231:753–760.
91.Smith TR,White AP. Narrowing of the proximal jejunal limbs at their passage through the transverse mesocolon: a potential pitfall of laparoscopic Roux-en-Y gastric bypass. AJR 2004;183:141–143.
92.Chevallier JM, Zinzindohoue F, Cherrak A, et al. [Laparoscopic gastroplasty for severe obesity. 300 cases, evaluation of the first 150.] [French] Presse Med 2000;29:1921–1925.
93.Wiesner W, Schob O, Hauser RS, Hauser M. Adjustable laparoscopic gastric banding in patients with morbid obesity: radiographic management, results, and postoperative complications. Radiology 2000;216:389– 394.
94.Rapp-Bernhardt U, Wolf S, Dohring W, Bernhardt TM. [Intragastric penetration as a local complication after performance of gastric banding.] [German] Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 2000;172:305–306.
95.Hainaux B,Coppens E,Sattari A,Vertruyen M,Hubloux G, Cadiere GB. Laparoscopic adjustable silicone gastric banding: radiological appearances of a new surgical treatment for morbid obesity. Abdom Imaging 1999;24:533–537.
96.Mortele KJ, Pattijn P, Mollet P, et al. The Swedish laparoscopic adjustable gastric banding for morbid obesity: radiologic findings in 218 patients. AJR 2001;177:77–84.
97.Van Rosendaal GM,Verhoef MJ, Mace SR, Kinsella TD. Decision-making and outcomes for percutaneous endoscopic gastrostomy: a pilot study. J Clin Gastroenterol 1997;24:71–73.
98.Laasch H-U, Wilbraham L, Bullen K, et al. Gastrostomy insertion: Comparing the options—PEG, RIG or PIG? Clin Radiol 2003;58:398–405.
99.Dinkel HP, Triller J. [Percutaneous gastrostomy with balloon PEG balloon replacement tubes by the radiologist. An interventional technique for simple placement of feeding catheters without surgical or endoscopic intervention.] [German] Radiologe 2001; 41:473–477.
100.Ryan JM, Hahn PF, Mueller PR. Performing radiologic gastrostomy or gastrojejunostomy in patients with malignant ascites. AJR 1998;171:1003–1006.
101.Dewald CL, Hiette PO, Sewall LE, Fredenberg PG, Palestrant AM. Percutaneous gastrostomy and gastrojejunostomy with gastropexy: experience in 701 procedures. Radiology 1999;211:651–656.
102.Funaki B, Peirce R, Lorenz J, et al. Comparison of balloonand mushroom-retained large-bore gastrostomy catheters. AJR 2001;177:359–362.
103.Funaki B, Zaleski GX, Lorenz J, et al. Radiologic gastrostomy placement: pigtailversus mushroomretained catheters. AJR 2000;175:375–379.
104.Reimer W, Farres MT, Lammer J. Gastric wall dissection as a complication of percutaneous gastrostomy. Cardiovasc Intervent Radiol 1996;19:288–290.
105.Kanterman RY, Hicks ME, Simpson KR, Malden ES, Picus D, Darcy MD. Nonsurgical management of gastric or duodenal perforation from a Wills-Oglesby- type gastrostomy tube. J Vasc Interv Radiol 1996;7: 737–741.
106.Pickhardt PJ, Rohrmann CA Jr, Cossentino MJ. Stomal metastases complicating percutaneous endoscopic gastrostomy: CT findings and the argument for radiologic tube placement. AJR 2002;179:735–739.
107.Fleischer DE, Van de Mierop F, Eisen GM, et al. A new system for defining endoscopic complications emphasizing the measure of importance. Gastrointest Endosc 1997;45:128–133.
108.Barragan Casas JM, Hernandez Hernandez JM, Garcinuno Jimenez MA, et al. Bacteremia caused by digestive system endoscopy. Rev Esp Enferm Dig 1999; 91:105–116.

3
Duodenum
Congenital Abnormalities
Duplication
As in the rest of the gastrointestinal tract, a duodenal duplication may or may not communicate with the true lumen. Most duplications are located along the first and second parts of the duodenum. An occasional one is discovered incidentally, but most descending duodenal duplications coming to medical attention are associated with pancreaticobiliary abnormalities. An occasional one obstructs the common bile duct; at times a bizarre biliary communication is identified. A duplication can appear as a cystic tumor in the periampullary region. Some are associated with duodenal compression and obstruction or recurrent acute pancreatitis, a presentation more common in children. Rare complications of noncommunicating duodenal duplications include necrosis and perforation resulting in an acute abdomen, becoming infected or even causing an intussusception.
Ultrasonography (US) reveals a hyperechoic inner layer and a hypoechoic outer muscular layer; these layers are better defined with endoscopic US. At times peristaltic activity is identified.
Cholangiopancreatography is helpful in defining any associated ductal anomalies. Often endoscopic retrograde cholangiopancreatography (ERCP) is not successful due to periampullary distortion and magnetic resonance cholangiopancreatography (MRCP) is neces-
sary. A barium study aids in detecting duodenal communication and in narrowing the differential diagnosis considerably. With duodenal communication, the differential includes a duodenal diverticulum and a necrotic tumor. Without duodenal communication, other intramural or extrinsic tumors are in the differential. The presence of stones suggests communication with pancreaticobiliary ducts. With some of these duplications, computed tomography (CT), US, and magnetic resonance (MR) do not establish a site of origin, although imaging should differentiate a duodenal duplication from a choledochal cyst and pancreatic pseudocyst. Also, the clinical presentation is different with the latter two entities. In either case, the underlying pancreaticobiliary ductal anatomy is often atypical and should be defined prior to resection or anastomosis.
Atresia/Stenosis/Web
Duodenal atresia, believed to be due to failure of duodenal recanalization early in gestation, is the most common cause of a high bowel obstruction in a neonate. The most common site for atresia is just distal to the papilla of Vater, and these neonates present with bilious vomiting, whereas the less common atresia proximal to the papilla mimics gastric outlet obstruction. Atresias range from partial to complete; the atretic segment is either string-like or appears as a web.
107
108
Table 3.1. Duodenal obstruction in the neonate
Midgut malrotation with volvulus
Duodenal atresia
Duodenal stenosis
Duodenal web
Ladd’s bands
Duodenal duplication
Annular pancreas
Anomalous portal vein anterior to duodenum
Tumor or distention in an adjacent structure
Not all neonatal duodenal obstruction is due to duodenal atresia or stenosis (Table 3.1). Midgut malrotation with volvulus in the newborn presents with complete duodenal obstruction and has a similar presentation.
Patients with duodenal atresia are prone to having other anomalies, including an annular pancreas and other pancreaticobiliary anomalies. A rare patient has an anomalous portal vein anterior to the duodenum. The prevalence of duodenal atresia is increased in Down syndrome, in a setting of congenital heart disease, and in those with a tracheoesophageal fistula. Esophageal atresia and duodenal atresia may coexist; in such a setting, unless a tracheoesophageal fistula is present, the stomach fills with fluid and distends. The large bowel is normal in these patients. A microcolon implies an additional, more distal obstruction.
A rare congenital duodenal obstruction in an infant leads to gastric emphysema (1).
An occasional adult develops a partial duodenal obstruction secondary to a web. Duodenal webs are associated with aspirin ingestion
(2) and long-term use of nonsteroidal antiinflammatory drugs (3).
Radiographs reveal no gas distal to the atresia. An exception is with a rare bifid common bile duct anomaly where the bile duct communicates both with proximal and distal duodenal segments.
After repair of duodenal atresia, the duodenal bulb continues to be larger in volume than usual.
Trauma
ADVANCED IMAGING OF THE ABDOMEN
wall thickening, at times to the point of obstruction. In its retroperitoneal position close to other major structures, duodenal trauma is commonly associated with injury to the adjacent pancreas and liver. An endoscopic biopsy is a cause of an intramural duodenal hematoma
(4). An intramural duodenal hematoma can develop secondary to pancreatitis. An abused child had both a duodenal hematoma and contained duodenal and proximal jejunal perforations (5); duodenal obstruction ensued and exploratory laparotomy found a calcified, fibrotic mesentery and both duodenal and jejunal strictures.
Full-thickness duodenal rupture does occur in patients sustaining blunt duodenal injury; the second duodenal segment is most often involved. Most of these ruptures are associated with other intraabdominal injuries, but one should keep in mind that traumatic retroperitoneal duodenal perforation initially reveals few clinical sign and symptoms. Blunt abdominal trauma can also result in diverticular perforation.
Imaging detection of an early duodenal perforation is difficult. For instance, among traumatic duodenal perforations studied with CT, extravasation of contrast is identified only in a small minority.
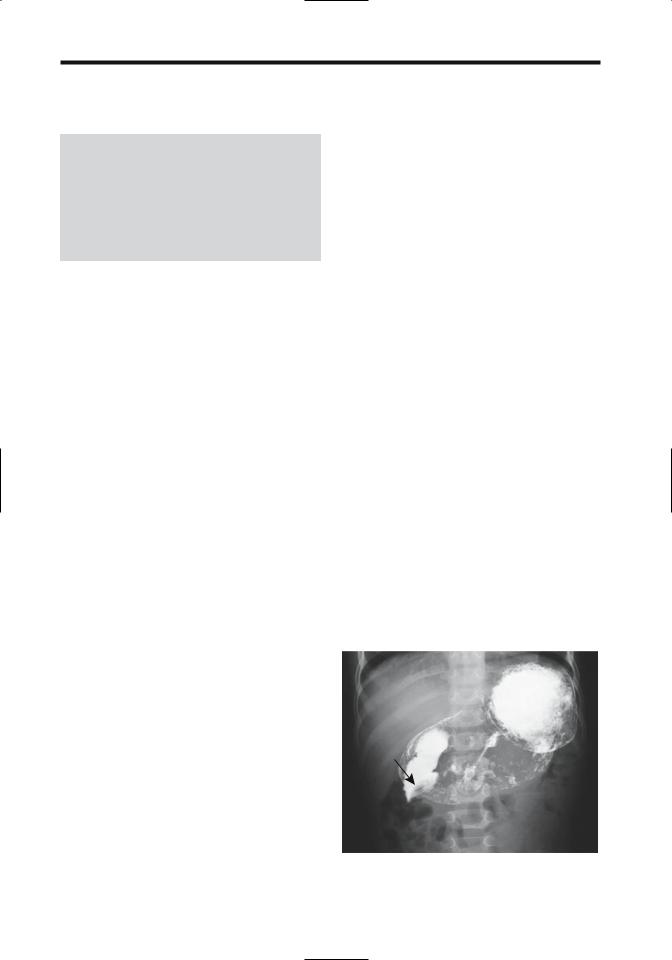
Blunt trauma is a common cause for a duodenal hematoma (Fig. 3.1). Imaging reveals duodenal
Figure 3.1. Duodenal hematoma (arrow) resulting in a highgrade obstruction. The obstruction resolved spontaneously.

109
DUODENUM
A veiled right kidney is a US finding of a retroperitoneal duodenal perforation (6).
A bullet causes not only duodenal but also adjacent organ damage. Fistulas are one sequela, at times to unusual sites such as a pyeloduodenal fistula, if damage is not recognized initially.
Wall Thickening
Infection/Inflammation
Some infections manifest primarily in the proximal small bowel, whereas others are detected distally. For convenience, most small bowel infections are discussed in Chapter 4.
Duodenitis
The term duodenitis implies duodenal inflammation and is a histologic diagnosis, although endoscopists often apply this term to a gross reddened mucosa believed to represent inflammation.
Myriad conditions thicken duodenal valvulae conniventes: infections, eosinophilic gastroenteritis, amyloidosis, mastocytosis, lymphangiectasia, infiltrating neoplasms, Crohn’s disease, and other entities. Ischemic duodenitis in the absence of prior surgery should suggest a small vessel vasculitis. Using thickened folds as a criterion for duodenitis, an upper gastrointestinal examination in children achieved a 46% sensitivity and 98% specificity for detecting duodenitis, compared to 54% sensitivity and 92% specificity for endoscopy (7); the low sensitivities were due mostly to normal findings for both radiology and endoscopy in children with mild duodenitis.
Peptic Ulcer Disease
The discovery of Helicobacter pylori has modified our understanding of peptic ulcer disease pathogenesis. Thus antral infection with H. pylori promotes antral gastritis, increased gastrin release, and increased acid production. Duodenal bulb H. pylori colonization promotes bulbar gastric metaplasia, leading to further H. pylori infection, more inflammation, and further inability to neutralize gastric acid load. In some patients this cycle persists until an ulcer
develops. Probably both bulbar gastric metaplasia and bulbar H. pylori colonization are necessary to develop a duodenal ulcer. Both the prevalence and the extent of gastric metaplasia and the amount of H. pylori in the duodenal bulb in patients with a duodenal ulcer are much higher than in those with a gastric ulcer or chronic gastritis. Eradication of H. pylori decreases the amount of acid entering the duodenum, and duodenal inflammation subsides.
Peptic ulcer disease is more prevalent in cirrhotic patients and those in chronic renal failure than in the general population. In cirrhotics, antral H. pylori colonization does not appear to play a major role in duodenal peptic ulcer disease.
Postbulbar duodenal peptic duodenitis is uncommon. At times a barium study reveals postbulbar ulcerations, thickened folds, or even a stenosis, and an endoscopic biopsy identifies inflammation; because peptic duodenitis here is uncommon, focal pancreatitis in an annular pancreas or celiac disease should be suspected and an endomysial antibody test obtained. In some patients an endoscopic biopsy revealed villous atrophy.
A barium study of peptic ulcer disease is usually straightforward. Ulcers in unusual locations and recurrent ulcers are more problematic. Peptic ulcer disease in younger children, although uncommon, does result in an unusual presentation and radiologic appearance. Ultrasonography reveals focal duodenal wall thickening, at times even an echogenic line adjacent to the ulcer, but US is rarely employed to detect duodenal ulcers.
Occasionally encountered is a giant duodenal ulcer; cocaine and methamphetamine use is associated with some of these giant ulcers, although many are idiopathic.
Some perforated duodenal ulcers result in a pneumoperitoneum and peritonitis. Other ulcers perforate into an adjacent structure, including pancreas, liver, bile ducts, and adjacent colon. Duodenocolic and duodenocholedochal fistulas secondary to peptic ulcer disease are rare. At times such a perforation clinically mimics myocardial ischemia; conventional radiographs should suggest a correct diagnosis.
Contrast-enhanced CT of a perforating ulcer reveals focal bowel wall thickening, enhancement, and inflammatory changes in surround-
110
ing tissues. A wall defect at the site of perforation is rarely detected. On the other hand, extraluminal gas bubbles, at times in Morison’s pouch, should be sought. A water-soluble upper gastrointestinal contrast study, although out of vogue in a number of institutions, remains a viable alternative.
Crohn’s Disease
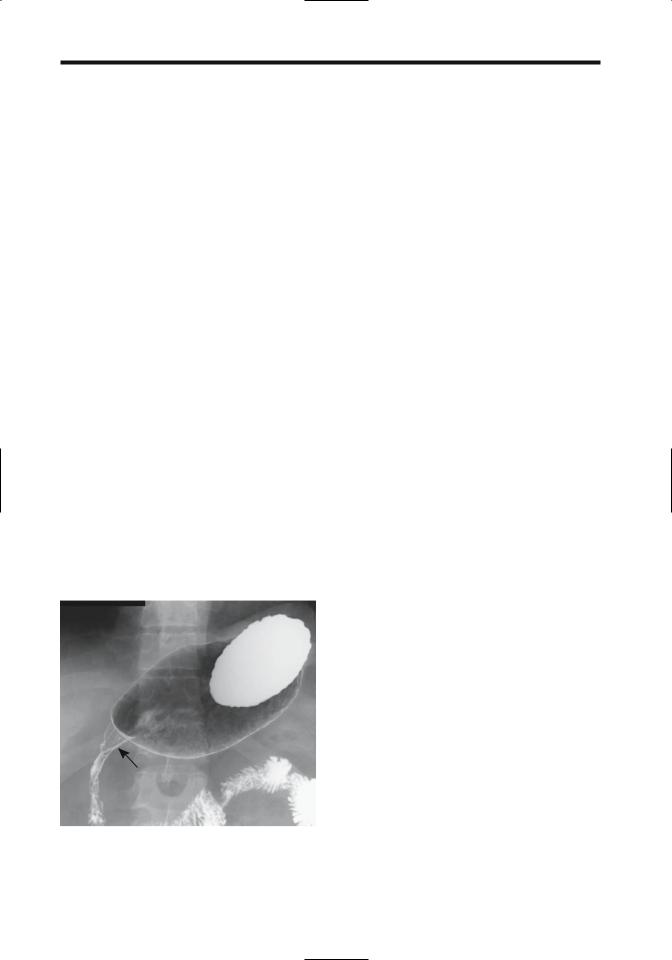
Duodenal Crohn’s disease is notoriously difficult to diagnose. A biopsy is often of little help, most often revealing nonspecific inflammation and only occasionally containing granulomas. Imaging findings include aphtha, a peptic ulcer-like appearance, fistula, a stenotic segment varying in length, or complete obstruction (Fig. 3.2). Presence of thickened folds may be the only clue to Crohn’s disease. Fistulas are less common than with ileal involvement. Most duodenocolic fistulas are secondary to colonic Crohn’s disease rather than duodenal Crohn’s. Occasionally duodenal deformity is secondary to Crohn’s disease in an adjacent loop of bowel. A double-contrast barium study should differentiate between primary and secondary duodenal involvement.
A number of patients with duodenal Crohn’s have undergone a Billroth I operation in the
ADVANCED IMAGING OF THE ABDOMEN
mistaken belief that their underlying deformity was secondary to peptic ulcer disease. Invariably inflammation and strictures progress.
Diverticulitis
Diverticula are more common in the duodenum than in the rest of the small bowel, yet duodenal diverticulitis is sufficiently rare to be a curiosity. Clinically, duodenal diverticulitis mimics peptic ulcer disease, cholecystitis, or pancreatitis. A number of patients have unsuspected duodenal diverticulitis first detected at exploratory laparotomy for an acute abdomen, an understandable event given the rarity of this condition.
A barium study should detect thickened duodenal folds secondary to duodenal inflammation. Computed tomography identifies any associated inflammation and abscess. Inflammation medial to the duodenal sweep suggests focal pancreatitis (and indeed adjacent portions of the pancreas tend to be inflamed). Perforations lead either to a focal walled-off cavity or peritoneal spill. Extraluminal gas can range from focal bubbles to pneumoperitoneum.
Most duodenal diverticula are peri-Vaterian in location and, when infected, surrounding inflammation thus is centered around the duodenopancreatic interface. Imaging differential diagnosis includes focal pancreatitis (at times suggesting an inflamed annular pancreas) or postbulbar ulcer disease.
Figure 3.2. Duodenal Crohn’s disease in a patient with known ileal disease. A duodenal stricture and effaced valvulae conniventes are evident (arrow). (Courtesy of Daniel Wopperer, M.D., Rochester, New York.)
Associated with Dialysis and Pancreatitis
Thickened duodenal folds are common in patients on dialysis. Although patients in renal failure have an increased prevalence of peptic ulcer disease, not all dialysis patients with thickened folds have clinical peptic ulcer disease.
Duodenal involvement is common in acute pancreatitis, even to the point of obstruction. Thickened folds are seen in a setting of chronic pancreatitis.
Tuberculosis
Even in endemic regions duodenal tuberculosis is rare, and this diagnosis is thus often not suspected.
Duodenal stenosis was discovered in a 69- year-old man who also had common bile duct

111
DUODENUM
obstruction due to stones (8); Crohn’s disease was initially suspected, and therapy with corticosteroids was instituted until tuberculosis was identified.
Sarcoidosis
The liver followed by the stomach are the most common abdominal organs involved. Small bowel sarcoidosis, including duodenal, is uncommon. Gastrointestinal tract sarcoidosis is one cause for a protein-losing enteropathy. Sarcoidosis involving the papilla can obstruct the common bile duct.
Radiographically, sarcoidosis mimics Crohn’s disease.
Amyloidosis
Duodenal amyloidosis is uncommon. Clinically, diffuse amyloid infiltration leads to lumen narrowing and, with extensive involvement, obstruction. Occasionally infiltration is polypoid in appearance.
Cystic Dystrophy
An unusual condition, duodenal cystic dystrophy is associated with chronic pancreatitis and presents as focal duodenal wall thickening. At times associated with heterotopic pancreatic tissue, and with about half the affected patients having chronic pancreatitis, the relationship of duodenal cystic dystrophy to duplication cysts is speculation. Most cystic dystrophy foci contain cysts, but an occasional one is solid. Some of these cystic structures suggest dilated ducts lined with exocrine pancreatic epithelium. A rare one evolves into an abscess. Occasionally a thickened duodenal wall progresses to a stricture. Whether this entity is simply inflammation of heterotopic tissue is conjecture. In either case, the spectrum consists of cysts, fibrosis, and possible stricture.
Imaging in patients with duodenal cystic dystrophy reveals a thickened duodenal wall and intramural cysts varying in size. Some of these cysts are >1cm in diameter, the thickened duodenal wall is isodense on precontrast CT and varies from hypovascular to exhibiting strong contrast enhancement (9). Duodenal cystic dystrophy appears mostly hypoechoic with US.
Necrosis
Caustic ingestion generally results in esophageal and, less often, gastric injury. Occasionally, however, it is associated duodenal injury, even necrosis. In general, contrast imaging is safe in these individuals.
Tumors
Nonneoplastic
Hyperplastic/Metaplastic
Hyperplastic or metaplastic duodenal polyps are uncommon. A rare metaplastic polyp occurs in the duodenal bulb and is associated with H. pylori, regressing after anti–H. pylori therapy.
Heterotopic Tissue
Heterotopic pancreatic tissue is common in the duodenal wall. This tissue contains acini and ducts, but not islet cells. Occasionally localized inflammation within such heterotopic tissue results in valvulae conniventes thickening and eventually evolves into a duodenal stricture.
Less often encountered in the duodenum is heterotopic gastric tissue. Both chief cells and parietal cells are present.
Hamartoma
Brunner gland hamartomas are most common in the first portion of the duodenum and most occur in middle-aged individuals. Although submucosal in origin, an occasional one becomes pedunculated. Some are several centimeters in size. They consist of proliferating Brunner gland tissue surrounded by fibrosis, fat, and lymphoid tissue. These hamartomas bleed, obstruct if they are large enough, or are simply an incidental finding.
Patients with untreated sprue develop multiple small polyps scattered throughout the duodenal bulb. These polyps probably represent Brunner’s gland hyperplasia, although they may represent an intrinsic abnormality of sprue.
An isolated myoepithelial hamartoma is rare in the duodenum; most of these occur in a setting of one of the polyposis syndromes. An isolated duodenal hamartomatous polyp occasionally also contains an adenocarcinoma.

112
Polyposis Syndromes
Polyposis syndromes are discussed in more detail in Chapter 5. Covered here are only those aspects pertinent to the duodenum.
Patients with familial adenomatous polyposis develop polyps in the stomach, duodenum, small bowel, and colon. True prevalence of duodenal polyposis in these patients is not known, because small adenomas are often not visualized either by imaging or endoscopy. Also, part of the confusion involves a definition of the term polyp; not all adenomas in familial polyposis protrude into the lumen. An endoscopic duodenal search of patients with familial adenomatous polyposis detects adenomas in 30% to 60% of patients; these tumors tend to be either flat or flat-topped elevations with a central depression. The most common duodenal location is periampullary. An occasional duodenal polyp is a villous adenoma.
These patients are at increased risk of developing a papilla of Vater carcinoma, and a duodenal adenocarcinoma is a not uncommon cause of death in familial polyposis patients who have had a previous colectomy. They are also prone to developing mesenteric fibromatosis.
Periampullary adenomas have been removed endoscopically, although the long-term results of such therapy are not known. Prophylactic pancreaticoduodenal resection has been performed for severe duodenal polyposis (10).
Bulbar Tumors
Primary duodenal bulb neoplasms are so rare that an average physician will not encounter one in a lifetime of practice. They are probably reportable. Only occasional descriptions exist of bulbar villous adenomas and adenocarcinomas.
An early bulbar cancer (well-differentiated papillary adenocarcinoma) was detected on a barium study as a smooth, sessile polyp (11). A very rare one develops in a setting of a chronic or refractory bulbar ulcer, but bulbar ulcers are not considered to be premalignant.
More common duodenal bulb neoplasms are benign mesenchymal tumors such as leiomyoma or lipoma. A rare metastatic melanoma mimics a benign ulcer; in some parts of the world resected skin tumors are not examined by
ADVANCED IMAGING OF THE ABDOMEN
a pathologist and thus a patient may not be aware of a prior melanoma resection.
Periampullary Tumors
Clinical
Discussed here are tumors originating from the papilla of Vater and adjacent duodenum. Bile duct tumors are covered in Chapter 8, and pancreatic origin tumors are discussed in Chapter 9.
Linguistic purists argue about correct usage, but the terms periampullary, papillary, and peri-Vaterian tumor are synonymously used in the literature and are descriptive terms for tumors of the mid-descending duodenum, papilla of Vater, pancreas adjacent to the papilla, sphincter of Oddi, and even distal common bile duct. Some malignant tumors infiltrate surrounding structures to the point that a specific site of origin cannot be identified. Nevertheless, the site of origin should be sought because of prognostic differences. Thus a carcinoma of the papilla of Vater is associated with a better prognosis than a carcinoma originating from adjacent pancreas.
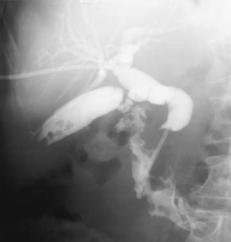
The two most common periampullary tumors are adenoma and adenocarcinoma. An adenoma, quite often villous, can be quite large and not cause biliary obstruction (Fig. 3.3). These adenomas are premalignant. Once jaundice ensues in a patient with a known peri-Vaterian adenoma, however, malignant degeneration and invasion is likely. An occasional peri-Vaterian carcinoma first manifests as pancreatitis. In general, a cancer originating at the papilla that has not spread through the sphincter of Oddi invades locally; on the other hand, a tumor penetrating through the sphincter tends to metastasize to regional lymph nodes. Also, a malignancy penetrating the duodenal muscularis propria generally has a poor prognosis.
Other papillary tumors are uncommon and an occasional one is difficult to classify. A papilla of Vater adenomyoma produced obstructive jaundice (12). A rare carcinosarcoma and a primary carcinoma containing both glandular and neuroendocrine differentiation have been reported at the papilla.
Patients with von Recklinghausen’s disease are prone to developing peri-Vaterian tumors,

113
DUODENUM
Figure 3.3. Peri-Vaterian villous adenoma (arrows). A focus of carcinoma can readily be present and thus a biopsy showing no cancer is of limited help with these tumors. (Courtesy of Arunas Gasparaitis, M.D., University of Chicago.)
some being rather uncommon, such as a somatostatinoma (13).A very rare peri-Vaterian tumor acts as a lead point for common bile duct prolapse (14).
Imaging
Even when small, some papilla cancers obstruct the bile ducts, yet neither endoscopy nor imaging is able to detect them. With further growth these tumors are readily identified with an imaging study or endoscopic cholangiopancreatography. An occasional periampullary carcinoma is not detected initially at ERCP but becomes evident as a protruding mass only after a papillotomy.
Peri-Vaterian carcinomas range in appearance from primarily polypoid and intraluminal, primarily intramural and infiltrating the duodenal wall, growing mostly into the pancreatic head, to those with a mixed growth pattern. Those growing into the pancreas are often indistinguishable from a cholangiocarcinoma or primary pancreatic carcinoma both by imaging and histology (Fig. 3.4).
Transabdominal US has a limited role in detecting these tumors.
A mostly intramural neoplasm has to be >1cm or so to be identified by endoscopic US. Nevertheless, of periampullary tumors requir-
A 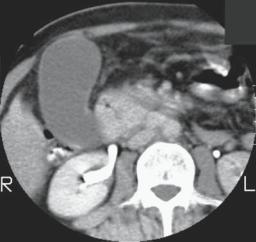
 B
B
Figure 3.4. A: Carcinoma of papilla. Contrast-enhanced CT reveals a nonenhancing tumor involving descending duodenum and pancreatic head (arrows). A distended gallbladder is evident. (Courtesy of Patrick Fultz, M.D., University of Rochester.) B: Carcinoma originating close to the papilla of Vater. Extensive bile duct and pancreatic invasion is present. Exact site of tumor origin could not be determined.
