

9 Cardiovascular imaging
Contents
Aortic anatomy 659
Acute aortic syndrome 661
Traumatic aortic injury 665
Aortic aneurysms and miscellaneous disorders 666
Coronary CT angiography 673
Cardiac MRI 678
Plain film imaging of heart disease 681
Nonischemic myocardial disease 686
Pericardial disease 689
658
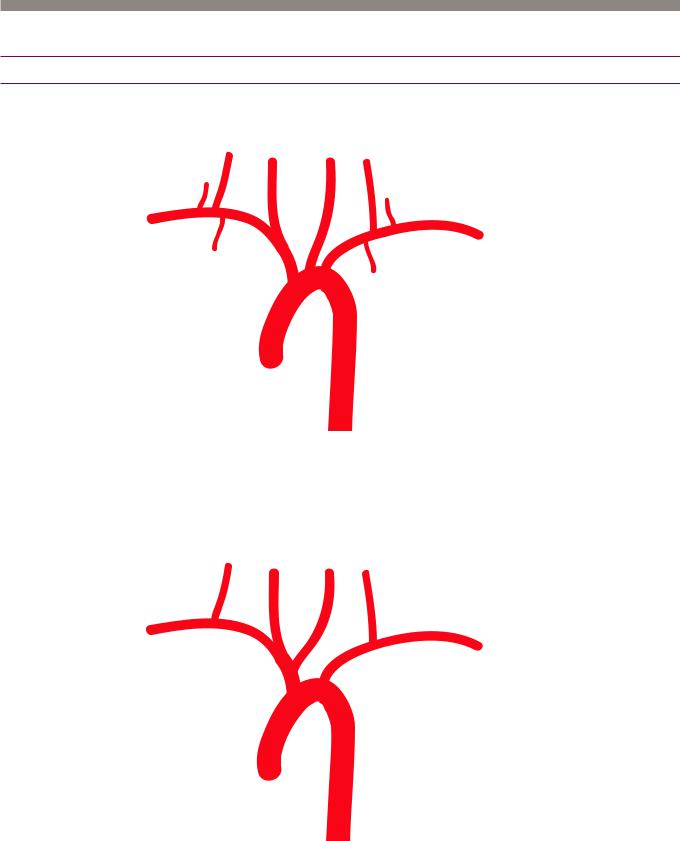
Aortic anatomy
Aortic arch and variants
Normal aortic arch branching
right |
right |
|
left |
|
||
common common |
left |
|||||
vertebral |
||||||
|
|
carotid |
carotid |
vertebral |
||
thyrocervical |
|
|
|
|
thyrocervical |
|
trunk |
|
|
|
|
||
|
|
|
|
|
trunk |
|
right |
|
|
|
|
|
|
subclavian internal |
|
|
|
|
left |
|
mammary |
|
|
|
|
||
brachio- |
|
|
subclavian |
|||
|
|
|
||||
|
cephalic |
aortic arch |
internal |
|||
|
|
|
||||
|
|
|
|
|
mammary |
|
ascending |
|
|
descending |
|||
aorta |
|
|
|
aorta |
||
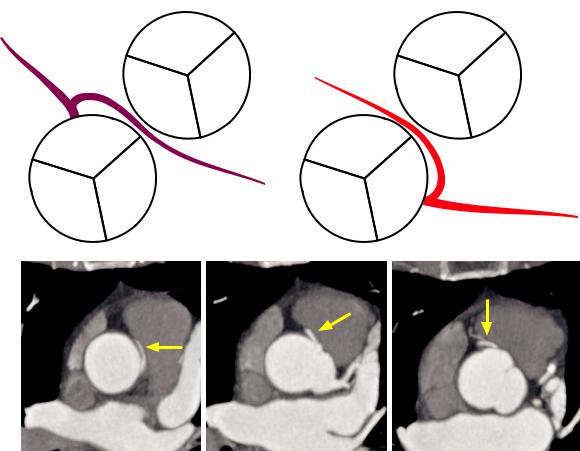
•The normal aortic branching pattern is seen 66% of the time and features three arteries arising from the aortic arch: Brachiocephalic trunk (inominate artery), left common carotid, and left subclavian artery.
Common origin of the brachiocephalic artery and left common carotid artery
right |
right |
left |
|
vertebral common common |
left |
||
|
carotid |
carotid |
vertebral |
right |
|
|
subclavian |
left |
|
brachio- |
||
subclavian |
||
cephalic |
|
|
|
aortic arch |
|
ascending |
descending |
|
aorta |
||
aorta |
||
|
•A common origin of the brachiocephalic artery and left common carotid artery is seen in 13% of patients (more commonly in blacks) and is often incorrectly referred to as a “bovine aortic arch.” The term “bovine aortic arch” is a misnomer and is not the preferred description of this anomaly, as a true bovine arch in cattle features a single great vessel arising from the aortic arch.
659

Aberrant right subclavian
right |
right |
left |
left |
vertebral |
common |
common |
|
|
carotid |
carotid |
vertebral |
right
subclavian  left subclavian
left subclavian
ascending |
descending |
|
aorta |
||
aorta |
||
|
•An aberrant right subclavian is seen in 1% of patients. The right subclavian artery arises directly from the aortic arch distal to the left subclavian and loops behind the esophagus on its way into the right arm.
•Itisveryuncommonforanaberrantrightsubclavianarterytocausesymptoms,butthis anomalymayrarelybeacauseofdysphagiaviaesophagealcompression,calleddysphagia lusoria.Bariumesophogramfeaturesaposteriorindentationontheesophagus.
Although usually incidental, it is important to mention the presence of an aberrant right subclavian artery when reading a neck CT. If thyroid surgery is planned, then the recurrent laryngeal nerve will not be in its usual location.
•A diverticulum of Kommerel represents a small bulge at the origin of the aberrant subclavian artery.
Left vertebral origin off aorta
right |
right |
left |
left |
vertebral common common vertebral |
|||
|
carotid |
carotid |
|
right |
|
|
subclavian |
|
left |
|
|
|
|
brachio- |
subclavian |
|
aortic arch |
|
|
cephalic |
|
|
|
|
|
ascending |
descending |
|
aorta |
|
|
aorta |
|
|
|
•Afourvesselarchwithdirectoriginofthevertebralarteryoffoftheaortaisseenin6%of thepopulation.Theleftvertebralisthethirdaorticbranch,proximaltotheleftsubclavian.
660
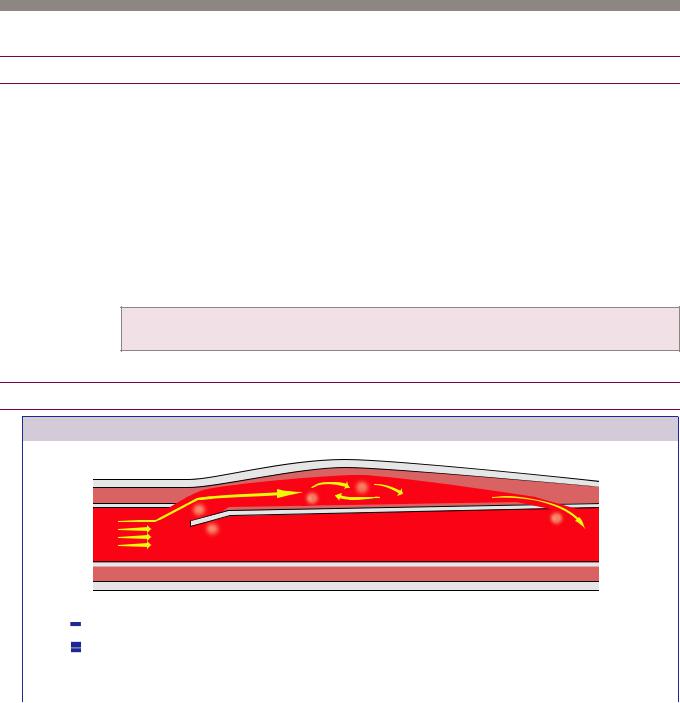
Acute aortic syndrome
Overview of acute aortic syndrome
Acute aortic syndrome represents a clinical spectrum of three related diseases that are characterized by damage to at least one component of the aortic wall, and presents as severe chest pain.
A defect primarily in the intima is seen in penetrating atherosclerotic ulcer (PAU).
A defect in the media only describes intramural hematoma (IMH).
A defect in the intima extending to media is the hallmark of aortic dissection.
A defect in all three layers (aortic transection) is almost always due to trauma and is
considered a separate entity.
The treatment of acute aortic syndrome depends primarily on the location (ascending
versus descending aorta) and is often the same regardless of the underlying etiology.
Ascending aorta Treatment is most commonly surgical.
Descending aorta Treatment is most commonly medical (blood pressure control).
maging of the full aorta is generally required in any acute aortic pathology.
Aortic dissection
The key feature of dissection is a disruption in the intima, which allows high-pressure
blood to infiltrate and expand the media.
The most common risk factor for aortic dissection is hypertension, but other risk factors include connective tissue disorders (especially Marfan syndrome), cocaine use, aortopathy associated with a bicuspid aortic valve, weight lifting, and sudden deceleration injury.
The classification of aortic dissection is the Stanford classification, which divides dissection into types A (ascending aorta) and B (non-ascending aorta). Type A is typically treated surgically, and type B is typically treated medically.
Aortic dissection secondary to atherosclerosis is more commonly type B (descending aorta).
661
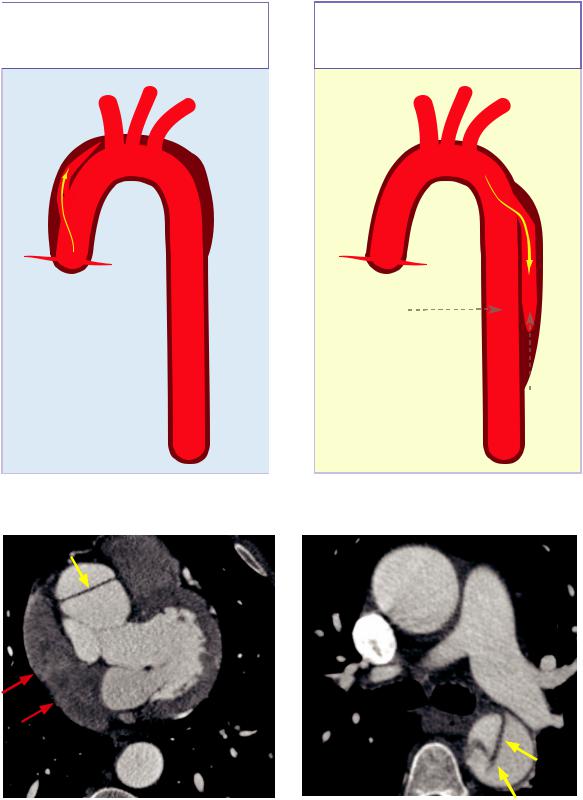
Classification of aortic dissection |
|
||||
|
|
|
|
|
|
|
Stanford A aortic dissection: |
|
Stanford B aortic dissection: |
||
|
Ascending aorta, ±descending aorta |
|
Descending aorta only |
||
|
Typically treated surgically |
|
Typically treated medically |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
true lumen
 false
false  lumen
lumen
Stanford A |
Stanford B |
Axial contrast-enhanced gated CT shows a dissection flap in the ascending aorta only
(yellow arrow). The descending aorta appears normal. There is a moderate hemopericardium consistent with rupture (red arrows).
Axial contrast-enhanced gated CT in a different patient shows a dissection flap in the descending aorta only (arrows).
662
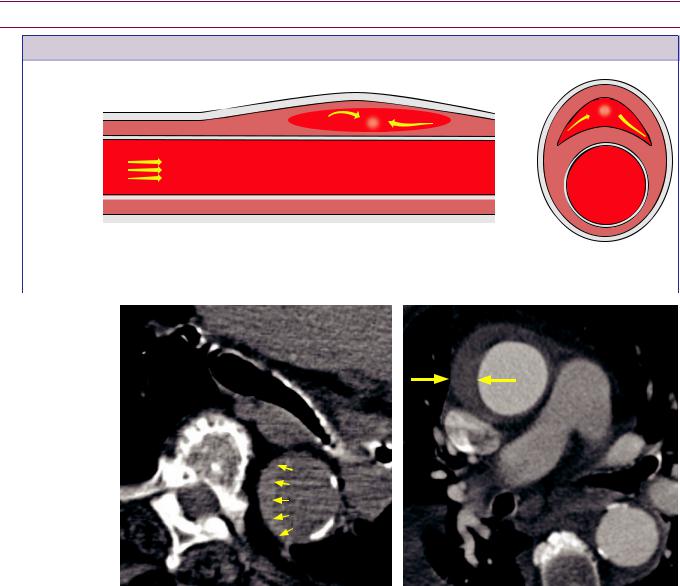
Intramural hematoma (IMH)

aortic lumen
aortic lumen
Rupture of vasa vasorum creates crescent-shaped hemorrhage within the media (in cross-sectional view, top right and below left). There may be associated aneurysm.
The intima is always intact. IMH can be thought of as a noncommunicating dissection.
ntramural hematoma on non-contrast CT: |
ntramural hematoma on contrast-enhanced |
Unenhanced CT shows very faint crescent |
CT: Contrast-enhanced CT in a different patient |
of hyperdense (50 HU) hemorrhage in the |
shows a crescentic hyperattenuating (50 HU) rim |
descending aorta (arrows). |
reflecting intramural hematoma (arrows). There is |
|
no disruption of the aortic lumen. |
ntramural hematoma (IMH) is a variant of dissection where blood collects within the media, without an intimal flap to connect the intramural hematoma with the aortic umen. IMH is thought to be due to rupture of the vasa vasorum, which are small blood vessels that supply the aortic wall.
Similar to dissection, IMH may be secondary to hypertension or trauma.
Clinically, IMH can present identically to aortic dissection with acute tearing back pain. The treatment recommendations for IMH are the same as dissection regarding involvement of the ascending versus descending aorta.
The key imaging finding of IMH is a faint peripheral hyperattenuating (45–50 HU) crescent within the aorta, best seen on noncontrast CT. In high-risk patients, the aortic dissection CT protocol typically includes an unenhanced CT prior to CTA to evaluate specifically for IMH. An alternative strategy in low-risk patients is to perform unenhanced CT only if there is suspicion of IMH on the CTA in order to decrease radiation exposure
663

Penetrating atherosclerotic ulcer (PAU)
|
adventitia |
|
|
media |
|
|
intima |
||
aortic lumen
Atherosclerotic plaque penetrates the intima.
Atherosclerotic plaque ulcerates, allowing blood to extend into the media. PAU may cause the media to enlarge, leading to an aneurysm formation.
Axial contrast-enhanced CT shows multiple penetrating ulcers (arrows) in the descending aorta, extending beyond the expected contour of the aortic lumen
Sagittalobliquegatedcontrast-enhancedCTina differentpatientshowsapenetratingatherosclerotic ulcer(arrow)oftheascendingaorta,extending beyondtheexpectedcontourofthelumen.
Penetrating atherosclerotic ulcer (PAU) is a focal defect in the intima that occurs at the site of an atherosclerotic plaque. PAU may lead to saccular aneurysm formation.
n contrast to dissection and IMH, PAU tends to be caused by atherosclerosis rather than hypertension. One of the theories of dissection secondary to atherosclerosis is that it begins as a penetrating ulcer.
On imaging, PAU appears as contrast ulcerating beyond the expected contour of the aortic wall. The primary differential would be a simple ulcerated atherosclerotic plaque, which would not extend beyond the expected contour of the aortic wall.
Atherosclerotic plaque represents chronic atherosclerotic disease and is not an acute aortic syndrome.
Multiple ulcers may be present throughout the thoracoabdominal aorta, and as with all acute aortic pathologies, imaging of the full aorta is recommended if a PAU is seen.
664

Traumatic aortic injury
Aortic trauma
Chest radiograph in a trauma patient shows indistinct widening of the mediastinum. The endotracheal tube is displaced to the right (arrow).
Contrast-enhanced CT shows a mediastinal hematoma and traumatic aortic dissection (arrow). Indistinctness of the lateral arch of the aorta is concerning for tear.
Catheter aortogram shows a large, fusiform |
Post repair aortogram shows stent graft in place |
pseudoaneurysm (arrows) of the aortic isthmus, |
with good opacification of the arch vessels. |
distal to the brachiocephalic artery. |
|
Aortic trauma: Case courtesy Michael Hanley, MD, University of Virginia Health System.
•There are three relatively fixed levels of the aorta where traumatic aortic injuries occur secondary to deceleration injury, which are the aortic root, isthmus, and hiatus. The resulting pseudoaneurysm is held in place by the surrounding connective tissues. In the small percentage of patients who survive blunt aortic trauma, injury occurs at the isthmus in 95% of cases.
Associated injury to the origins of the brachiocephalic, left carotid, or left subclavian arteries is frequently present.
•CTA is the gold standard for evaluation of suspected traumatic aortic injury.
•Traumatic mediastinal hemorrhage is often venous; however, aortic injury is the primary concern. Direct CT signs of traumatic aortic injury include a dissection flap, pseudoaneurysm, and intramural hematoma.
Mediastinal hemorrhage that is separated from an intact aorta by a fat plane can be presumed to be venous, and treatment is conservative. In contrast, hemorrhage in contact with the aortic wall is suggestive of aortic injury and necessitates surgical treatment.
665
Aortic aneurysms and miscellaneous disorders
Thoracic aortic aneurysm
Thoracic aortic aneurysm (TAA)
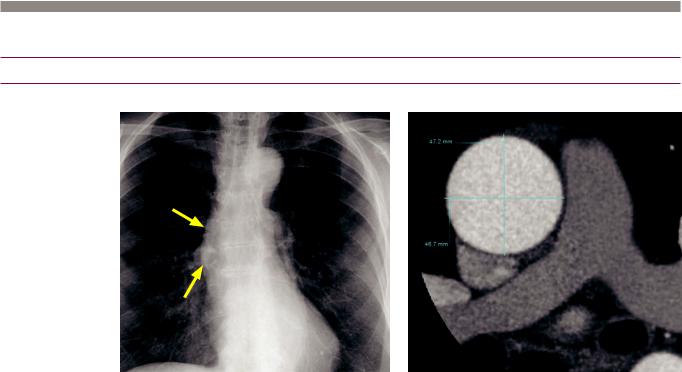
Thoracic aortic aneurysm: Frontal chest radiograph demonstrates ectasia of the right aortic border (arrows). Gated contrast-enhanced CT with a double-oblique short-axis reformation demonstrates an ascending aortic diameter of 4.7 cm, representing an aneurysm.
Case courtesy Michael Steigner, MD, Brigham and Women’s Hospital.
•A thoracic aortic aneurysm (TAA) is defined as an ascending aortic diameter >4 cm or a descending thoracic aorta >3 cm in diameter. Aortic size may also be normalized to body surface area and compared to reference values.
•Double-oblique short-axis multiplanar reformatted images perpendicular to the aortic lumen (i.e., true short-axis) should always be used to measure aortic diameter.
•Most thoracic aortic aneurysms are caused by atherosclerosis, with the descending thoracic aorta affected more commonly. Almost one third of patients with atherosclerotic TAA will have an associated abdominal aortic aneurysm.
•Non-atherosclerotic causes of TAA include connective tissue disorders (such as Marfan and Ehlers–Danlos syndromes), bicuspid aortic valve (BAV) associated aortopathy, vasculitis (including Takayasu arteritis, giant cell arteritis, ankylosing spondylitis, and relapsing polychondritis), cystic medial necrosis, and infectious aortitis.
•Annuloaortic ectasia represents dilated sinuses of Valsalva and ascending aorta with effacement of the sinotubular junction, resulting in a tulip bulb-shaped aorta. Annuloaortic ectasia is associated with Marfan and Ehlers–Danlos syndromes.
•Surgical treatment is recommended for an ascending TAA >5.5 cm in diameter and a descending TAA >6 cm in diameter. However, patients with connective tissue disorders and BAV aortopathy (meeting criteria for valve replacement) have a lower surgical threshold of 4.5 cm. Beyond simple size criteria, annual growth rate >1 cm/year (or >5 mm/6 months) is an indication for surgical repair.
•A sign of impending rupture is the draped aorta sign, which describes drooping of the posterior aorta against the spine on an axial image.
•Complications of TAA treatment include rupture, dissection, infection, endoleak, and paraplegia (caused by artery of Adamkiewicz occlusion).
666

Abdominal aortic aneurysm
Abdominal aortic aneurysm (AAA)
Abdominal aortic aneurysm: Axial CT of the infrarenal aorta demonstrates a large, peripherally calcified abdominal aortic aneurysm (arrows) containing extensive mural thrombus.
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Abdominalaorticaneurysm(AAA)isrelativelyprevalentinoldermen(seeninupto 5.9%ofmenbyage80)andlesscommoninwomen.Ruptureofabdominalaortic aneurysmisthe13thleadingcauseofdeathinoldermen.Riskfactorsfordevelopment ofanabdominalaorticaneurysmincludeage,malesex,smoking,andfamilyhistory.
•An abdominal aortic aneurysm is defined as an aortic diameter ≥3 cm. Similar to measurement of thoracic aortic aneurysms, double-oblique reformatted images should be used to obtain a true cross-sectional diameter. Volume measurement can also be used to monitor for endoleak on follow-up.
•The natural history of abdominal aortic aneurysm is progressive enlargement and eventual rupture.
The annual risk of rupture for an AAA between 5.5 and 5.9 cm is 9.4%.
The annual risk of rupture for an AAA between 6.0 and 6.5 cm is 10.2%.
The annual risk of rupture for an AAA between 6.5 and 6.9 cm is 19.2%.
The annual risk of rupture for an AAA greater than 7 cm is 32.5%.
•Ultrasoundscreeningofhigh-riskpatientsisapprovedbyMedicareintheUSforpatients olderthanage65.Ifananeurysmisdetectedonscreening,follow-upisrecommended:
Aneurysm <4 cm: Follow-up in 6 months; if no change g annual surveillance.
Aneurysm 4–4.5 cm: Follow-up in 6 months; if no change g 6-month surveillance.
Aneurysm 5–5.5 cm: Consider surgery.
Aneurysm >5.5 cm: Surgery recommended.
•In addition to a size >5.5 cm, repair is recommended when the AAA is expanding at a rapid rate (>5 mm/year) or is symptomatic.
•The mortality of elective open AAA repair is >3%, while the mortality for urgent repair is 19%. A ruptured AAA has a mortality of at least 50%.
•Repair of abdominal aortic aneurysm can be performed with a traditional open or endovascular technique. Endovascular repair is preferred for patients with high surgical risks and is associated with reductions in major morbidity and hospital time. Long-term outcomes are equivalent between endovascular and open repair, but endovascular repair often requires repeat interventions.
•Complications of endovascular repair of AAA include rupture, dissection, infection, endoleak, and aorto-enteric fistula.
667
Abdominal aortic aneurysms - Endoleaks
•An endoleak is persistent flow into an excluded aneurysm sac after endovascular treatment with a stent graft.
Type I endoleak: Inadequate seal of graft
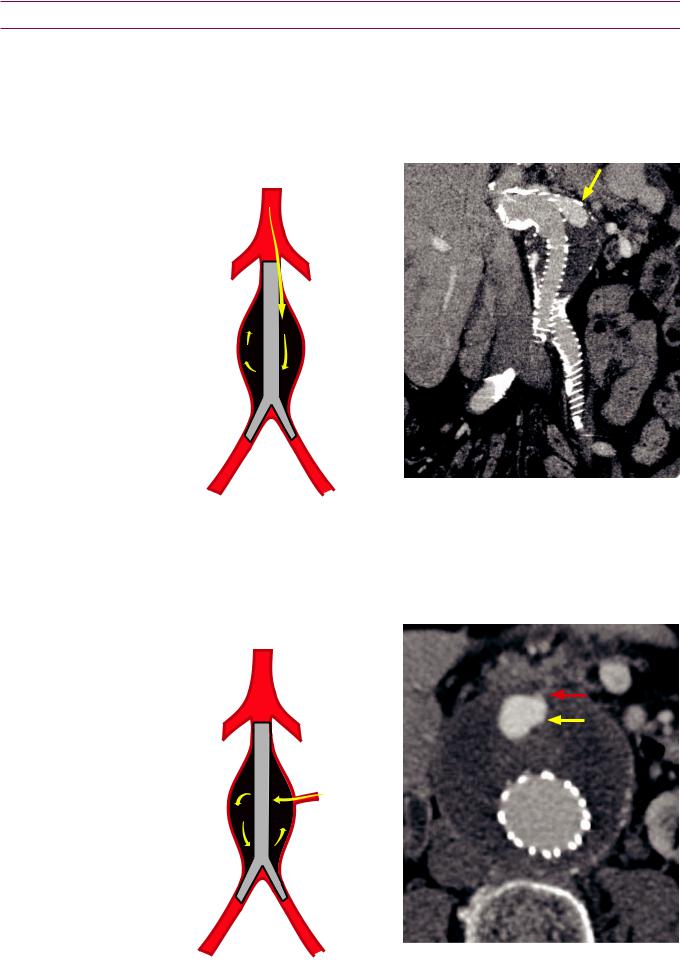
•Type I endoleak is inadequate graft seal. Type IA is a proximal leak and IB is distal.
Type IA endoleak: Inadequate proximal seal |
Inadequate proximal seal leads to enhancement |
allows blood into the excluded aneurysm sac. |
in the proximal aneurysm sac (yellow arrow). |
Type II endoleak: Persistent collateral flow to excluded aneurysm
•Type II endoleak is persistent collateral flow to the excluded aneurysm sac, which typically arises from the lumbar arteries or the inferior mesenteric artery (IMA).
Type II endoleak: Communication from either the IMA or a lumbar artery causes blood to flow into the excluded aneurysm sac.
Axial image shows focal enhancement in the excluded aneurysm sac (yellow arrow), with faint communication (red arrow) leading to the IMA.
668

Type III endoleak: Device failure causing leakage
•Type III endoleak represents device failure causing leakage through graft fabric or segments of a modular graft.
Type III endoleak: Blood enters the excluded |
Coronal CT demonstrates contrast extravasating |
aneurysm sac via a defect in the graft. |
(yellow arrow) through the angulation of a |
|
modular aortic graft. |
Types IV and V endoleaks
•Both type IV and type V endloeaks are diagnoses of exclusion as no endoleak can be visualized by imaging although the sac continues to increase in size.
•Type IV endoleak: Type IV endoleak is caused by a porous graft and is typically transient and seen intraprocedurally. Type IV endoleak usually resolves within one month after withdrawal of anticoagulation. It is rarely seen with modern grafts.
•Type V endoleak: Also called endotension, type V endoleak is continued expansion of the aneurysm without any other
endoleak present, thought to be due to an endoleak below the resolution of imaging.
669

Miscellaneous aortic disorders
Aortitis
Active aortitis: T1-weighted post-contrast fat saturated MRI demonstrates circumferential mural thickening and enhancement of the aortic wall (arrows).
Case courtesy Michael Steigner, MD, Brigham and Women’s Hospital.
•Aortitis is inflammation of the aorta, which may be either infectious or inflammatory.
•A complication of infectious aortitis is the development of a mycotic aneurysm.
Mycotic aneurysm due to Staphylococcus aureus: Sagittal (left image) and coronal (right image) contrast-enhanced CT shows marked periaortic inflammatory change (red arrows) associated with a saccular aortic aneurysm (yellow arrows).
•Inflammatory aortitis can be due to Takayasu arteritis, giant cell arteritis, ankylosing spondylitis, polyarteritis nodosa, rheumatoid arthritis, and immune complex disease. Inflammatory aortitis is treated with corticosteroids.
•The acute phase of aortitis will show circumferential mural thickening and enhancement. There may be an associated aneurysm, dissection, or intramural hematoma. In contrast to intramural hematoma, aortitis tends to cause circumferential thickening rather than the eccentric, crescentic thickening of IMH.
MRI findings of active aortitis include an aortic wall thickness >2 mm and enhancement of the aortic wall.
•In the chronic phase of the disease, there can be long segmental stenoses and/or aneurysms.
670
Takayasu arteritis
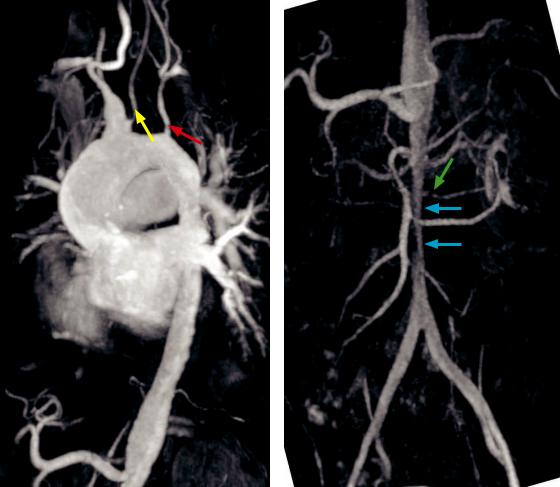
Takayasu arteritis:
Sagittal-oblique maximum intensity projection MR angiogram (left image) of the aortic arch shows narrowing of the left common carotid artery (yellow arrow) and left subclavian artery (red arrow). Note the incidental common origin of the brachiocephalic trunk and the left common carotid artery.
Coronal maximum intensity projection MR angiogram (right image) of the abdominal aorta in the same patient shows a long smooth stenosis of the infra-celiac abdominal aorta (blue arrows), with a focal stenosis of the accessory left renal artery (green arrow).
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Also known as pulseless disease, Takayasu arteritis is an idiopathic, inflammatory, large-vessel vasculitis that involves the thoracic and abdominal aorta, subclavian arteries, carotid arteries, pulmonary arteries, and large mesenteric arteries.
•Takayasu arteritis typically affects young to middle-aged women.
•On imaging, long smooth stenoses are classic. Imaging is often indistinguishable from giant cell arteritis, with the patient’s age being the main distinguishing factor. Takayasu arteritis occurs in relatively younger patients and giant cell arteritis is rare in patients under age 50.
•During the acute phase, treatment is with steroids. If symptomatic stenoses occur, endovascular treatment can be performed, but only when the active inflammation has resolved, as measured by normalization of the erythrocyte sedimentation rate.
671
Aortic coarctation
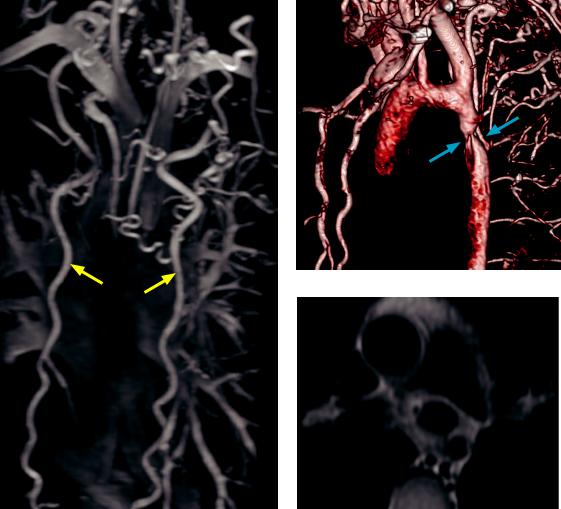
Aortic coarctation: Coronal maximum intensity projection MR angiogram (left image) shows extensive collateral vessels throughout the thorax, with prominent internal thoracic arteries (yellow arrows). 3D volume rendered CT of the aorta (top right image) demonstrates the coarctation (blue arrows) distal to the aortic isthmus. Double inversion recovery fast spin echo MRI (bottom right image) also demonstrates the coarctation.
Case courtesy Michael Steigner, MD, Brigham and Women’s Hospital.
•Aortic coarctation is congenital focal narrowing of the proximal descending aorta.
•The adult form of coarctation is usually juxtaductal (at the junction of the ductus arteriosus), leading to upper extremity hypertension. In contrast, an infant presenting with congestive heart failure due to coarctation is usually due to a preductal variant, which functions as a left ventricular obstructive lesion.
•In the setting of coarctation, prominent collaterals develop between the internal thoracic arteries to both the epigastric vessels and intercostal arteries.
•The radiographic findings of coarctation include the 3 sign of the left upper heart border, which represents a double bulge from the focal aortic narrowing and poststenotic dilation. Rib notching is frequently seen from collateral intercostal vessels.
•Phase contrast MRI can be used to measure the gradient of flow across the coarctation. Calculation of the flow differential between the proximal descending aorta and the aorta at the hiatus aids in determining hemodynamic significance.
•A pseudocoarctation represents focal narrowing of the aorta, similar in morphology to a true coarctation; however, there is no pressure differential and thus no collaterals.
672

Coronary CT angiography
Evidence for using coronary CT to evaluate for ischemic cardiac disease
•Coronary CT angiography (CCTA) is an excellent test to rule out hemodynamically significant coronary artery disease. Meta-analyses of multiple trials have shown the negative predictive value for CCTA to be in the high 90s.
•The ACRIN-PA and ROMICAT-II clinical trials (both published 2012 NEJM) evaluate the use of CCTA in the emergency room setting foracute chest pain in low to medium risk patients. Both trials conclude that early CCTA improves efficiency, clinical decision making, and leads to a shorter hospitalization, although the ROMICAT-II trial did note increased radiation exposure in the CCTA group and no significant cost difference.
•Coronary CT is very sensitive for hemodynamically significant (>50% lumenal diameter) stenoses; however, a stenosis found on CT may be overcalled, especially if there is calcified plaque, which can cause a blooming artifact.
ECG gating and radiation dose
•ECG gating is used to minimize cardiac motion. The choice of ECG gating has a large effect on patient radiation dose.
To estimate the radiation dose, the dose–length product (DLP) should be multiplied by a conversion factor of 0.017 to arrive at the dose in millisieverts.
•In a retrospectively gated exam, continuous CT scanning is performed throughout the cardiac cycle and the images are correlated to the ECG cycle afterwards.
The main advantage of retrospective gating is the ability to create cine reconstructions to evaluate cardiac and valvular function, typically with 10–20 frames per cardiac cycle.
The main disadvantage of retrospective gating is a significant increase in radiation exposure compared to a prospectively gated study.
•Forprospectivegating,theECGisusedtotimeimageacquisitionataspecificphaseofthe cardiaccycle,exposingthepatienttoradiationonlyduringthissegmentofthecardiaccycle.
The main advantage of prospective gating is decreased radiation exposure.
However,sinceonlyafractionofthecardiaccycleisacquired,cinereconstructionsarenotpossible.
Spatial resolution and grading of stenoses
•Coronary arteries have an average luminal diameter of approximately 3 mm.
•CT has an isotropic voxel resolution of 0.35 to 0.5 mm, which allows only 6 to 9 voxels to image the entire coronary artery lumen. This is insufficient resolution to grade a stenosis with accuracy greater than approximately 20% of the diameter.
•In contrast, catheter angiography has a spatial resolution of approximately 0.16 mm, to depict the average lumen of a coronary artery over approximately 18 pixels.
•Due to the limited spatial resolution of CT, a stenosis is classified into categories: <20%; 20–50%; 50–70%, or >70%. A >50% stenosis is considered potentially hemodynamically significant. Conversely, a <50% stenosis is considered not hemodynamically significant.
Temporal resolution and “freezing” of cardiac motion
•ACTscannerrequiresslightlymorethan180degreesofgantryrotationforimage acquisition:Foracompleterotationtimeof330ms,thetemporalresolutionis~175ms.
•DualsourceCThastwoX-raysourcesoriented90degreestoeachotherandneedstorotate only90degreestocompleteareconstruction,withatemporalresolutionof~75ms.
Procedure
•Alowheartrate(typicallybelow60bpm)isdesiredinordertomaximizetheR–Rinterval.
673
Beta-blockadeisusuallynecessarytoachievethistargetheartrate.Oralmetoprololis administered(between5and25mg,typicallyadministeredin5mgdoses).Oralbeta blockergivesbetterheartratecontrolanddecreasedheartratevariabilitycomparedtoIV.
Just prior to scanning, sublingual nitroglycerin (0.5–0.8 mg) is administered to dilate
the coronary arteries.
Coronary artery anatomy
|
|
SVC |
Aorta |
|
|
|||||||||
posterior |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
LMCA |
|
|
||||
R |
L |
|
|
|
|
|
|
|
LCx |
|||||
|
|
|
|
|
|
|
|
|
|
|
||||
anterior |
|
SAN |
|
Pulm Art |
LAD Ramus |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
RCA |
conus |
|
|
||||||||
|
|
|
branch |
|
|
|||||||||
|
|
|
|
|
|
|
||||||||
RCA, right coronary artery |
|
|
|
|
OM |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
SAN, SA nodal branch |
|
|
|
|
acute |
|
|
|
|
|
|
|
Diag |
|
PDA, posterior descending artery |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
marginal |
|
|
|||||||||
PLA, posterolateral artery |
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
AVN, AV nodal branch |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
AVN |
Diag |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
LMCA, left main coronary artery |
|
|
|
|
|
|
|
|
|
|
|
Septal |
||
|
|
|
|
|
|
|
|
PLA |
|
|
|
|||
LAD, left anterior descending |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LCx, left circumflex |
|
|
|
|
|
|
|
|
|
|
|
|
Diag 3 |
|
OM, obtuse marginal |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diag, diagonal |
|
|
RCA |
|
|
|
|
|
|
|
|
|
|
|
|
|
PDA |
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||
(continues
posterior)
LAD
(continues
posterior)
The diagram above is in anatomic orientation, as if one were looking directly at the patient from the front.
Coronary artery origination
Most commonly, there are two coronary artery origins off the proximal aorta at the sinuses of Valsalva. The most common anomaly is a high take-off from the sinotubular junction or above.
There are three coronary sinuses. The right coronary artery arises from the right coronary sinus (located
anterior); the left main |
|
anterior |
right |
|
|
|
|
||
coronary artery arises from |
R |
L |
|
|
the left coronary sinus |
|
left |
||
|
|
non- |
||
(located left-posterior); and |
|
|
|
|
|
posterior |
coronary |
|
|
no coronary artery arises from |
|
|
|
|
|
|
|
|
|
the noncoronary sinus (located |
|
|
|
|
right-posterior). |
|
|
|
|
674
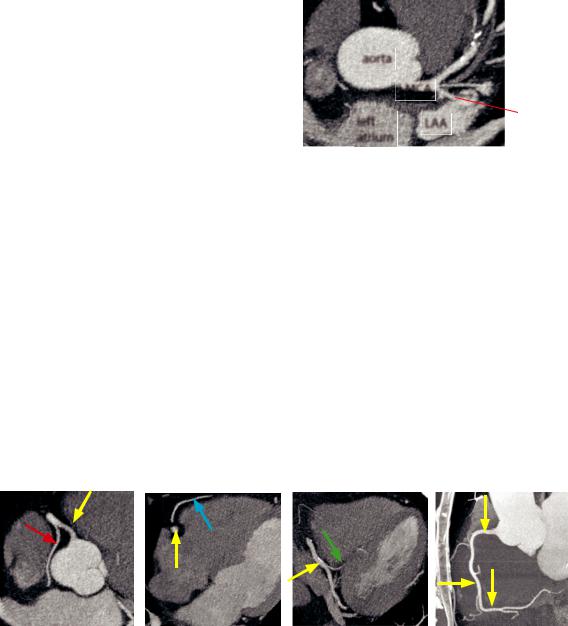
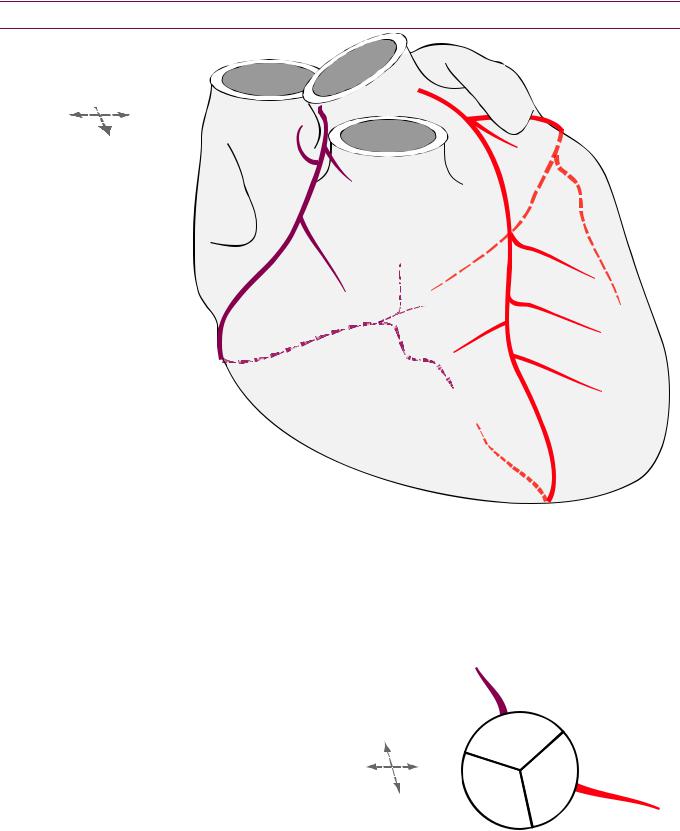
Left main coronary artery (LMCA)
The left main coronary artery (LMCA) courses between the pulmonary artery and the left atrial appendage (LAA).
The LMCA bifurcates into left anterior descending (LAD) and left circumflex (LCx) arteries. A ramus branch may be present to
form a trifurcation.
Left anterior descending (LAD) coronary artery
aorta |
|
|
|
LAD |
|
|
|
|
|
|
LMCA |
|
|
ramus |
|
|
|
||
left |
LAA |
|
|
LCx |
|
|
|
||
atrium |
|
|
|
|
The leftanteriordescending(LAD)coronaryarterycoursesintheanteriorinterventricular
groove,whichistheanatomicgroovebetweentherightandleftventricles.
The LADgivesoffthediagonalbranches(LAD Diagonal)andseptalbranches,which penetratetheinterventricularseptumtosupplybloodtotheanteriorhalfoftheseptum.
Left circumflex (LCx) coronary artery
The left circumflex (LCx) coronary artery courses underneath the left atrial appendage
(LAA) in the left atrioventricular groove (between the left ventricle and left atrium).
The LCx artery gives off the obtuse marginal (OM; circOMflex) branch, which supplies the posterolateral wall of the left ventricle. The angle of the lateral wall of the obtuse marginal artery is obtuse, hence the name.
TheLCxuncommonly(~7%)suppliestheposteriordescendingartery(PDA),whichisthe criteriaforaleft-dominantsystem.Intheanatomicdiagramonthepreviouspage,themore commonright-dominantsystemisshown,wheretherightcoronaryarterysuppliesthePDA.
Right coronary artery (RCA)
Axia |
Axia |
Axia |
Sagitta |
RCA(yellowarrow)arises |
RCA(yellowarrow)gives |
RCA (yellow arrow) |
Sagittal thick-slice MIP |
fromtherightcoronary |
offacutemarginal(blue |
gives off PDA (green |
shows the complete |
cuspandgivesofftheSA |
arrow),whichcourses |
arrow) in this right- |
course of RCA (yellow |
nodalbranch(redarrow). |
alongtheanteriorRVwall. |
dominant system. |
arrows). |
The right coronary artery (RCA) mirrors the LCx in course, sitting within the right
atrioventricular groove.
The first branches of the RCA are the conal branch, anteriorly (supplies the right
ventricular outflow tract) and the sinoatrial node branch posteriorly.
The RCAgivesoffanacutemarginalbranch,whichcoursesanteriortotheright
ventricular(RV)freewallandmuscularbranchestosupplytheRVfreewall(notdrawn).
The atrioventricular node branch (AVN) branches off at the crux (junction of all four
chambers where the atrioventricular and interventricular grooves intersect).
The posterior descending artery (PDA) arises from the RCA in approximately 85% to
supply the posterior half of the ventricular septum.
The terminal branch of the RCA is most commonly the posterolateral artery (PLA) that
supplies the posterior left ventricle.
675
Determination of dominance
•Whichever side supplies the PDA, PLA, and AV nodal branch (AVN) is considered the dominant coronary artery. Most commonly, in about 85% of cases, the RCA is the dominant artery.
•Left-dominant anatomy is uncommon, occurring in approximately 7% of cases. In leftdominant anatomy, the LCx supplies the PDA, PLA, and AVN.
•Codominant anatomy can also be seen in approximately 7% of cases, typically with the RCA supplying the PDA, while the LCx supplies the PLA.
Structural coronary artery anomalies
Coronary artery anomalies: Overview
•Anomaliesofthecoronaryarteriesarerare.Amalignant coronaryarteryanomalycarries anincreasedriskofsuddendeath(inupto40%ofpatients),oftenassociatedwithexercise.
•Multidetector CT is the best modality to evaluate anomalous coronary artery anatomy.
•Anomalies of coronary artery origin may be always malignant (if arising from the pulmonary artery) or potentially malignant (depending on the course):
Coronary artery arising from the pulmonary artery which is malignant. Either the right or left main coronary artery may arise from the pulmonary artery. Both are very rare.
Anomalous left coronary artery from the pulmonary artery (ALCAPA).
Anomalous right coronary artery from the pulmonary artery (ARCAPA). RCA arising from left coronary sinus.
Left main coronary artery (LMCA) arising from right coronary sinus.
Left circumflex (LCx) or left anterior descending (LAD) arising from right coronary sinus. Any artery arising from the noncoronary sinus.
•Anomalies of coronary artery course may be benign or malignant and include:
Interarterial course (between the aorta and pulmonary artery) of an anomalous coronary artery is malignant.
Retroaortic, prepulmonic, and septal coronary artery course are all considered benign.
•An intramural course of a coronary artery is seen when the vessel courses through the wall of the aorta for a short segment. This anomaly is associated with sudden death. There is typically a slit-like configuration of the coronary artery on CCTA. The treatment of intramural coronary artery is bypass, reimplantation, or the unroofing procedure, which opens and enlarges the ostium from inside the aorta.
Benign coronary artery anomaly
|
Prepulmonic (benign) course of the left |
|
anterior descending (LAD) coronary |
|
artery: |
PA |
Axial image from a gated coronary CT |
|
shows an anomalous course of the LAD, |
|
which runs anterior (arrows) to the |
|
pulmonary artery (PA). |
|
Case courtesy Michael Hanley, MD, |
|
University of Virginia Health System. |
•An aberrant coronary artery course is considered clinically benign if the coronary artery does not course between the aorta and the pulmonary artery.
676
Malignant coronary artery anomaly
Malignant: Anomalous origin of LEFT coronary artery arising from the RIGHT coronary sinus, passing between the aorta and the PA.
Malignant: Anomalous origin of RIGHT coronary artery arising from the LEFT coronary sinus, passing between the aorta and the PA.
pulmonary |
pulmonary |
artery (PA) |
artery (PA) |
aorta |
aorta |
|
|
right |
|
non- |
non- |
left |
|
||
coronary |
coronary |
|
RVOT
Malignant course of anomalous right coronary artery:
SequentialaxialimagesfromacoronaryCTshowanomalousoriginoftherightcoronaryartery(arrow)from theleftcoronarysinus,whichthencoursesbetweentheaortaandtherightventricularoutflowtract(RVOT).
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Anomalousinterarterial(betweenthepulmonaryarteryorrightventricularoutflow tractandtheaorta)courseofacoronaryarterycarriesahighriskofsuddendeathofup to40%,associatedwithexercise.Itisthoughtthatdilationoftheaortaoccursduring exercise,whichmaycompresstheanomalousvesselresultinginmyocardialinfarction.
•Either an anomalous left coronary artery or anomalous right coronary artery may take a malignant interarterial course.
•Treatment is surgical bypass grafting.
Anomalous left coronary artery from the pulmonary artery (ALCAPA)/Bland–White–Garland syndrome
•Also called Bland–White–Garland syndrome, anomalous left coronary artery from the pulmonary artery (ALCAPA) is a very rare but serious coronary artery anomaly, where the left coronary artery arises from the pulmonary artery. Most affected patients are infants, with over 90% mortality in the first year of life if untreated.
•Treatmentissurgical,witheitherdirectimplantationoftheanomalouscoronaryartery(in children)orligationoftheanomalousvesselinconjunctionwithbypassgrafting(inadults).
•Lesscommonly,theRCAmayarisefromthepulmonaryartery,whichisknownas anomalousrightcoronaryarteryfromthepulmonaryartery(ARCAPA).Treatmentissimilar.
677

Myocardial bridging
•Myocardial bridging describes a band of myocardium overlying a segment of a coronary artery, most commonly seen in the mid LAD.
•Myocardial bridging is usually asymptomatic; however, it may be a cause of angina, myocardial infarction, or even death.
•If bridging is present and thought to be the source of the patient’s symptoms, further evaluation is recommended with exercise myocardial perfusion.
Cardiac MRI
Overview of cardiac MRI
•Cardiac MRI provides high-resolution dynamic imaging of the heart using primarily segmented steady-state free precession (SSFP) and/or gradient echo cine sequences.
•SSFP is a “white blood” sequence that provides excellent contrast between myocardium and blood pool. It is well-suited for cardiac MRI due to its high temporal resolution and excellent contrast.
•Cine MRI provides quantitative assessment of cardiac morphology and function. Realtime images can be used for subjective analysis in poor gating situations.
•Tissue characterization is typically performed with double or triple inversion fast spin echo sequences.
Contrast-enhanced perfusion MRI
•First-pass contrast-enhanced perfusion MRI is performed pre and post vasodilator stress to evaluate myocardial perfusion. Normal myocardium enhances, while areas of decreased perfusion will be relatively hypoenhancing.
Delayed contrast-enhanced MRI (DE-MR)
•Delayed contrast-enhanced MRI (DE-MR) is used to image changes in the myocyte to interstitial space ratio. This ratio decreases most commonly after a myocardial infarction where myocytes are replaced with scar tissue.
•In distinction to contrast-enhanced perfusion described above where first-pass enhancement is normal, any delayed enhancement in DE-MR is abnormal and represents an increase in the extracellular volume fraction.
•Although delayed enhancement is most commonly due to prior infarct, abnormal enhancement may also be due to nonischemic etiologies, depending on the pattern of enhancement.
•Normal myocardium is nulled out with an inversion time of approximately 300 milliseconds and therefore appears black, although the inversion time varies with delay time, excretion rates, contrast relaxicity, and volume.
Delayed enhancement: Ischemic cardiomyopathy
•Delayed enhancement post infarction will extend from the subendocardium to the epicardium in a vascular distribution.
Subendocardial delayed enhancement
•Since the endocardium is the most susceptible to ischemia, ischemic-type delayed enhancement will always involve the subendocardial surface and should have a vascular territory. After remodelling, a chronic infarct will demonstrate delayed enhancement.
678

Transmural delayed enhancement
Transmural delayed enhancement due to infarct:
Delayed contrast-enhanced short-axis cardiac MRI shows transmural enhancement (arrows) of the inferior wall of the left ventricle, consistent with transmural
infarction in the right coronary artery territory.
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Ischemic-type delayed enhancement may involve the endocardium only or may be transmural. Transmural enhancement extends across the entire myocardial thickness.
•Transmuraldelayedenhancementrepresentsnonviablescarfrompriortransmuralinfarct.
•Evaluation of the cine images will usually demonstrate hypokinesis in the region of abnormal delayed enhancement.
Delayed enhancement: Nonischemic
•Delayed enhancement not following a vascular territory is not due to ischemia. Several distinct patterns of nonischemic delayed enhancement have been described.
Mid-myocardial delayed enhancement
Mid-myocardial delayed enhancement due to Chagas disease:
Delayed contrast-enhanced short-axis cardiac MRI shows patchy mid-myocardial enhancement (arrows) throughout the left
ventricle myocardium. This is a nonischemic distribution, but is nonspecific.
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Dilated cardiomyopathy(DCM)isthemostcommonnonischemiccardiomyopathy.Most commonlyidiopathic,DCMmayalsobecausedbyalcoholabuse,myocarditis,ordrug toxicity.MRIimagingofDCMwillshowmid-myocardialdelayedenhancement.Additional MRIfindingsofDCMincludediffusechamberenlargementandreducedejectionfraction.
•Sarcoidosis isasystemicdiseaseofnoncaseatinggranulomaswithcardiacmanifestations ofarrhythmias,leftventriculardysfunction,andrestrictivecardiomyopathy.Cardiac findingsareusuallyseeninconjunctionwithothermanifestationsofsarcoid,including lungdiseaseandadenopathy.CardiacMRIofsarcoidtypicallyshowseithermidmyocardialorsubepicardialdelayedenhancementinanodularorpatchypattern.
•Chagas disease is caused by the protozoan Trypanosoma cruzi, and can lead to a cardiomyopathy. On cardiac MRI, there is typically epicardial or mid-myocardial delayed enhancement, which can be seen even before the development of symptoms.
•Hypertrophic cardiomyopathy is characterized by abnormal left ventricular myocardial thickening without dilation. Pathologic thickening may be diffuse or focal. In severe cases, hypertrophic cardiomyopathy may be a cause of sudden death. On cardiac MRI, there can be mid-myocardial delayed enhancement in the regions of hypertrophied myocardium and at the junctions of the interventricular septum and the right ventricular free wall, due to myofibril disarray. Evaluation of the cine images will show reduced diastolic filling of the left ventricle.
679
Epicardial/subepicardial delayed enhancement
Myocarditis isinflammationofthemyocardium,whichmaybesecondarytomultiple causes.Viralinfectionisthemostcommoncauseofmyocarditis,followedbyautoimmune disordersanddrugtoxicity.Inadditiontosubepicardialdelayedenhancement,cardiacMRI ofmyocarditisalsoshowswallmotionabnormalitiesintheaffectedregions.
Chag disease may cause epicardial or mid-myocardial delayed enhancement.
Sarcoidosis may cause either mid-myocardial or subepicardial delayed enhancement in a nodular or patchy pattern.
Circumferential subendocardial delayed enhancement
Amyloidosis is a disorder of glycoprotein deposition throughout the extracellular spaces. In the heart, amyloidosis causes biventricular myocardial thickening, which eads to diffuse ventricular subendocardial delayed enhancement.
Cardiac transplant patientsmaydemonstratecircumferentialsubendocardialdelayed enhancement,thoughttocorrelatewiththepresenceofmyocardialfibrosispathologically.
Summary of delayed enhancement MRI
Delayed enhancement MRI
subendocardial infarct |
transmural infarct |
Mid-myocardial |
Epicardial |
Circumferential |
dilated cardiomyopathy |
myocarditis |
amyloidosis |
myocarditis |
sarcoidosis |
systemic sclerosis |
sarcoidosis |
Chagas disease |
cardiac transplantation |
Chagas disease |
|
|
hypertrophic cardiomyopathy right ventricular pressure overload
680

Plain film imaging of heart disease
•By applying a systematic approach, the standard chest radiograph can offer valuable information about the presence or severity of heart disease.
•The radiographic evaluation of heart disease begins with an assessment of the size of the cardiac silhouette. Radiographically evident heart disease can be divided into two categories: Those with a normal-sized cardiac silhouette and those with an enlarged cardiac silhouette.
•Aratioofthecardiacsilhouettetotheinnerdiameterofthethorax(thecardiothoracic ratio)of0.55orgreater(onaPAprojection)suggestsenlargementofthecardiacsilhouette.
•Cardiovascular diseases with an enlarged cardiac silhouette include cardiomyopathy secondary to congestive heart failure, valvular regurgitation (aortic, mitral, or tricuspid regurgitation), high-output or volume overload states, dilated cardiomyopathy, pericardial effusion, and paracardiac mass.
•To distinguish between the various causes of enlarged heart cardiac disease, the key structures to evaluate are the left atrium and the aorta.
Iftheleftatriumisenlargedandthecardiacsilhouetteisenlarged,thatsuggestsmitralregurgitation. If the aorta is enlarged and the cardiac silhouette is enlarged, that suggests aortic regurgitation. If neither the left atrium nor the aorta is enlarged, that suggests one of the other etiologies.
•Cardiovascular diseases with a normal-sized cardiac silhouette include valvular stenosis (aortic or mitral stenosis), pulmonary artery hypertension, hypertrophic cardiomyopathy, restrictive physiology, and acute myocardial infarction.
•Similartoevaluationofdiseaseswithanenlargedcardiacsilhouette,thekeystructuresto evaluateinthepresenceofanormalcardiacsilhouettearetheleftatriumandtheaorta.
If the left atrium is enlarged and the cardiac silhouette is normal, that suggests mitral stenosis.
If the aorta is enlarged and the cardiac silhouette is normal, that suggests aortic stenosis or aortic aneurysm.
If neither the left atrium nor the aorta is enlarged, that suggests one of the other etiologies.
•Other structures to always evaluate include the pulmonary vascularity, thoracic wall, chamber enlargement, and the great vessels (pulmonary arteries, ascending aorta, aortic arch, and descending aorta).
Right ventricular enlargement
•The right ventricle is the most anterior cardiac chamber. Right ventricular enlargement causes displacement of the cardiac apex in a leftward direction (in contrast to left ventricular enlargement, which causes displacement in a left-inferior direction).
•Right ventricular enlargement may cause opacification of the retrosternal clear space on the lateral radiograph.
Right atrial enlargement
•The right atrium forms the right heart border. Right atrial enlargement causes lateral bulging or elongation of the right heart border.
Left ventricular enlargement
•The left ventricle forms the left heart border. Left ventricular enlargement typically causes displacement of the cardiac apex in a left-inferior direction.
•Note that hypertrophic cardiomyopathy does not cause enlargement of the external contour of the ventricle.
681
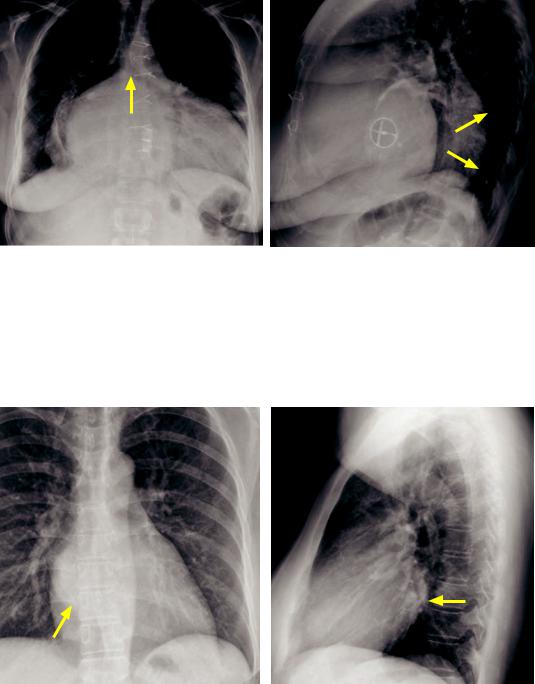
Left atrial enlargement
•The left atrium is the most posterior cardiac chamber. An enlarged left atrium may be caused by mitral regurgitation (with an enlarged cardiac silhouette) or mitral stenosis (with a normal cardiac silhouette).
•An enlarged left atrium can splay the carina, seen on the frontal view. On the lateral radiograph, an enlarged left atrium can elevate the left upper lobe bronchus.
Left atrial enlargement with cardiomegaly (due to mitral regurgitation): Frontal chest radiograph (left image) demonstrates massive cardiomegaly with marked enlargement of the left atrium, as evidenced by splaying of the carina (arrow). Lateral radiograph shows marked posterior displacement of the esophagus (arrows). A prosthetic mitral valve is present.
Case courtesy Ritu Gill, MBBS, Brigham and Women’s Hospital.
•An important clue to the presence of left atrial enlargement is the double density sign, seen over the right heart. The double density represents the right aspect of the enlarged left atrium visualized through the right atrium.
Left atrial enlargement without cardiomegaly (due to mitral stenosis): Frontal chest radiograph (left image) demonstrates the double density sign (arrow) with the left atrial border visualized through the right atrium. Lateral radiograph demonstrates posterior protrusion of the left atrium (arrow).
Case courtesy Ritu Gill, MBBS, Brigham and Women’s Hospital.
•On a lateral esophogram, an enlarged left atrium can displace the esophagus posteriorly and may be a cause of dysphagia.
682

Imaging and complications of myocardial infarction
Plain film evaluation of myocardial infarction (MI)
•The majority of initial plain chest radiographs obtained in patients with acute myocardial infarction (MI) are normal; however, the most common abnormality seen is increased pulmonary venous pressure (or overt pulmonary edema).
The presence of pulmonary edema after MI is a poor prognostic indicator.
Papillarymuscleruptureduetomyocardialinfarctiontypicallyproducesacutepulmonaryedema.Aclassic radiographicfindingisisolatedrightupperlobepulmonaryedemaduetoacutepapillarymusclerupture.
•The plain chest radiograph can evaluate for some complications of acute MI such as pericardial effusion, left ventricular aneurysm, and papillary muscle rupture.
•Inapatientwithacutechestpain,theinitialchestradiographcanoftensuggestan alternativecauseforthepatient’sacutechestpain,suchaspneumothoraxorpneumonia.
True left ventricular aneurysm
•A true left ventricular aneurysm is a focal outpouching of the ventricular wall, with all layers of the muscular wall affected.
•True aneurysms are associated with occlusion of the left anterior descending coronary artery.
•Themostcommonlocationofatrueleftventricularaneurysmisalongtheanterolateral orapicalwalloftheleftventricle.PlainfilmfindingsofatrueLVaneurysmincludean abnormalcontouralongthemidportionoftheleftcardiacborderneartheapex.
•A true aneurysm may calcify.
•True ventricular aneurysms are associated with wall motion dyskinesia but they rarely rupture. Management is medical.
False aneurysm (pseudoaneurysm)
•A false cardiac aneurysm (pseudoaneurysm) is a contained ventricular rupture, with only pericardial adhesions preventing a complete rupture. There is no myocardium in the wall of a false aneurysm.
•Falseaneurysmsareassociatedwithocclusionofthecircumflexorrightcoronaryarteries.
•Themostcommonlocationofafalseaneurysmistheupperdiaphragmaticandposterior wall.PlainfilmfindingssuggestiveofafalseLVaneurysmincludearetrocardiacdensity seenonthefrontalviewandanabnormalposteriorcontouronthelateralradiograph.
•False aneurysms may rupture at any time. An increase in size over sequential films is especially worrisome for impending rupture.
•Treatment of a left ventricular pseudoaneurysm is surgical.
Dressler syndrome
•Dressler syndrome is an autoimmune pericarditis, often associated with pericardial and pleural effusions.
Valvular disease
Endocarditis
•Endocarditisisinfectionofthecardiacvalves.Riskfactorsfordevelopmentofendocarditis includeintravenousdrugabuse,poordentalhygiene,diabetes,andprostheticvalves.
•Valvular vegetations are usually diagnosed by echocardiography, although CT angiography is routinely able to depict vegetations >1 cm in diameter. CT can also evaluate for the presence of a perivalvular abscess and assess for extracardiac complications of endocarditis, such as septic pulmonary emboli.
683
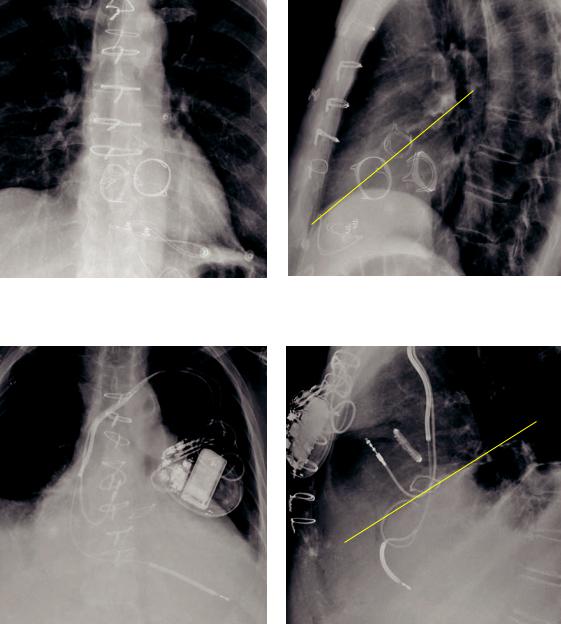
Imaging of prosthetic valves
A A
T M T M
Prosthetic aortic (A), mitral (M), and tricuspid (T) valves. Note that on the lateral radiograph (right image) the aortic valve is located on a plane drawn from the sternal/diaphragmatic junction and the carina (yellow line).
P
P A
A T
T
Prosthetic aortic (A), pulmonic (P), and tricuspid (T) valves in a different patient. The tricuspid prosthesis is a ring annuloplasty. The aortic valve is seen on the plane connecting the sternal/ diaphragmatic junction with the carina on the lateral radiograph.
•On the lateral radiograph, the aortic valve is centered on the plane drawn from the sternal/diaphragmatic junction and the carina.
•The tricuspid valve is to the right and anterior to the mitral valve.
•The pulmonic valve is the most superior and most leftward valve.
•Evaluation of prosthetic valves can be challenging in patients with abnormal chamber enlargement or cardiac rotation.
•The atrioventricular valves (mitral and tricuspid) are open in diastole.
684
Mitral regurgitation
•Acute mitral regurgitation secondary to myocardial infarction can present as acute pulmonary edema with a normal size heart.
•Chronic mitral regurgitation can lead to enlargement of the cardiac silhouette and left atrial enlargement.
The left atrial appendage (LAA) is often enlarged in patients with rheumatic disease; however, the LAA is typically not enlarged in nonrheumatic mitral regurgitation.
Mitral stenosis
•Mitral stenosis appears as a normal size heart with left atrial enlargement.
•Pulmonary venous pressures are typically elevated.
Mitral annular calcification
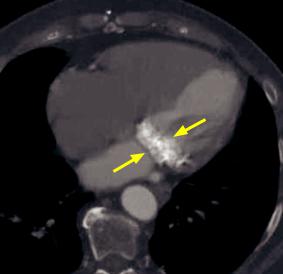
Mitral annular calcification:
Contrast-enhanced axial CT in bone windows demonstrates extensive calcification of the mitral valve annulus (arrows).
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Mitral annular calcification (MAC) is a degenerative process where calcium is deposited along the fibrous annulus encircling the mitral valve.
•MAC may be associated with increased risk of stroke, adverse cardiovascular events, and atrial fibrillation. MAC is considered a risk marker for cardiovascular disease.
•MAC can be associated with mitral regurgitation, but unlike mitral valve calcifications, MAC is not associated with mitral stenosis.
Aortic stenosis
•Aortic stenosis causes left ventricular hypertrophy; however, the heart size does not change.
•The ascending aorta is usually enlarged in long-standing aortic stenosis.
•The pulmonary vascularity is typically normal.
Aortic regurgitation
•Long-standing aortic regurgitation causes left ventricular enlargement, which is apparent on radiographs as cardiomegaly. There is typically enlargement of the ascending aorta.
•Similar to aortic stenosis, the pulmonary vasculature is typically normal.
Right-sided valvular disease
•The right side of the heart is preferentially involved in patients with carcinoid disease, leading to tricuspid and pulmonic valve dysfunction, although the left-sided valves can be involved as well.
685
Nonischemic myocardial disease
Catecholamine induced (takotsubo) cardiomyopathy
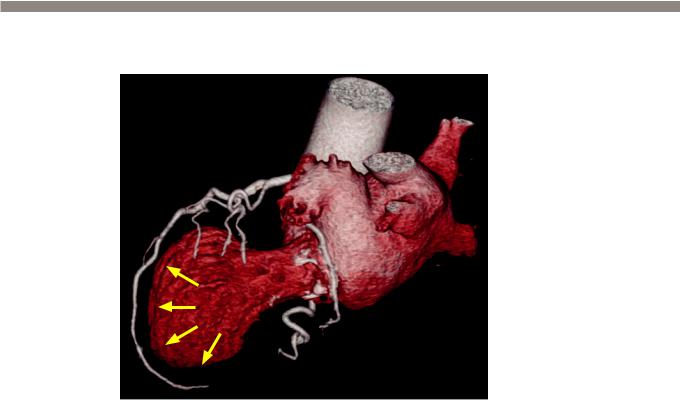
Catecholamine induced cardiomyopathy:
3D volume rendered image from a cardiac CT (with the myocardium removed) demonstrates marked
ballooning of the left ventricular apex (arrows). The coronary arteries are normal.
Case courtesy Michael Steigner, MD, Brigham and Women’s Hospital.
•Catecholamine induced cardiomyopathy, also known as takotsubo cardiomyopathy and broken heart syndrome, can clinically mimic acute myocardial infarction.
•Typically affecting older women in the setting of acute emotional stress, catecholamine induced cardiomyopathy can present with chest pain, abnormal ECG, and elevation of cardiac enzymes. Cardiac catheterization is normal.
One theory is that men may suffer catecholamine induced cardiomyopathy as well but typically don’t survive. There may be a protective effect of estrogen.
•Catecholamine induced cardiomyopathy is typically self-limited.
•On cardiac MRI or coronary CT, there is a characteristic ballooning of the cardiac apex. The shape of the heart is similar to a Japanese octopus pot, hence the name takotsubo. There is no abnormal delayed enhancement on MRI.
Arrhythmogenic cardiomyopathy
•Arrhythmogenic cardiomyopathy (previously called arrhythmogenic right ventricular dysplasia, as it was thought to only affect the right ventricle) represents fibrofatty replacement of ventricular myocytes, causing focal contraction abnormalities and/or aneurysm formation.
•Diagnosis is usually difficult and depends on major and minor criteria from EKG, imaging, and biopsy findings, as determined by task force in 2010. Imaging plays a supportive role in the diagnosis. Imaging findings may contribute 1 major and 1 minor criterion based on the presence of right ventricular enlargement or presence of focal aneurysm. The presence of myocardial fat is no longer in the criteria as fat can be seen in normal individuals with aging.
•Left ventricular involvement can be seen in up to three quarters of patients.
•Patients may suffer lethal arrhythmias and therefore require ICD placement once diagnosis is confirmed.
686
Myocardial noncompaction
•Myocardial noncompaction is a developmental defect in embryologic formation of the left ventricle, due to failure of part of the left ventricle to form a solid myocardium.
•Patients with noncompaction have an increased risk of adverse cardiac events, including arrhythmias, thrombus formation, stroke, and cardiomyopathy.
•On imaging, the left ventricle appears as heavily trabeculated as the right ventricle with a relatively thin left ventricular wall.
Hypertrophic cardiomyopathy (HCM)
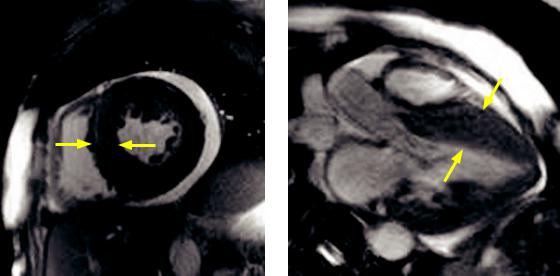
Hypertrophic cardiomyopathy: Short-axis (left image) steady-state free precession MRI shows concentric hypertrophy of the left ventricular myocardium (yellow arrows), without chamber enlargement. A small pericardial effusion is present. Three-chamber view (right image) from the same study better shows the septal predominance of the ventricular hypertrophy (arrows).
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Hypertrophic cardiomyopathy (HCM) is an autosomal dominant cardiomyopathy, characterized by hypertrophic left ventricular myocardium. HCM is the most common cardiomyopathy.
•The asymmetric septal hypertrophy variant, known as idiopathic hypertrophic subaortic stenosis (IHSS), may cause left ventricular outflow tract obstruction. Criteria for diagnosis include a wall thickness of ≥15 mm and a ratio of ≥1.5 compared to the lateral wall. A wall thickness ≥30 mm is an indication for ICD placement.
•Systolic anterior motion of the anterior leaflet of the mitral valve can cause mitral regurgitation and resultant left atrial enlargement.
•Although diagnosis is usually made by echocardiography, indications for MRI are to confirm the diagnosis of HCM, to measure the left ventricular mass, and to quantify the degree of subvalvular stenosis.
687

Restrictive cardiomyopathy
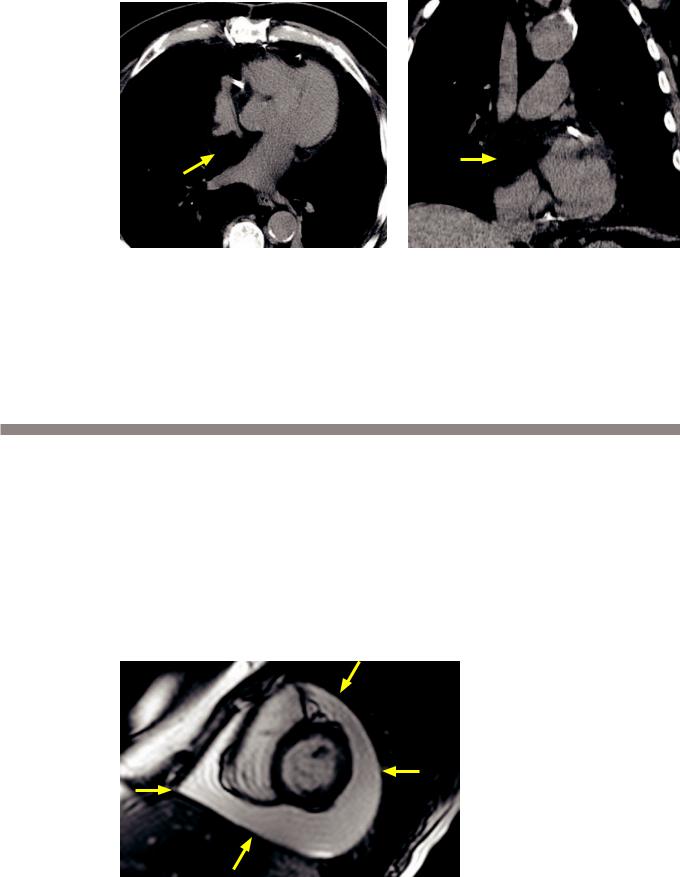
Impaired diastolic filling: Contrastenhanced CT demonstrates dilation and reflux of contrast into the IVC and hepatic veins (arrow), indicative of impaired right ventricular filling and resultant dilation of the right atrium, IVC, and hepatic veins.
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Restrictive cardiomyopathy is characterized by small, stiff, thickened ventricles that impair diastolic filling. This results in dilated atria and ultimately a dilated IVC. The etiology of the restrictive cardiomyopathy may be idiopathic or due to sarcoidosis, hemochromatosis, or myocardial deposition diseases (e.g., amyloidosis).
•Note that restrictive cardiomyopathy and constrictive physiology are different entities, although both conditions may feature identical ventricular pressure tracings and both are characterized by impaired diastolic filling.
•Constrictive physiology (subsequently discussed under pericardial disease) is secondary to increased pericardial pressure from thickened (often calcified) pericardium or pericardial effusion, causing impaired diastolic filling.
•The main role of imaging the heart in impaired diastolic filling is to exclude constrictive pericarditis as the etiology of the diastolic dysfunction. Constrictive pericarditis can be treated surgically by removing the pericardium; however, there is no effective treatment for restrictive cardiomyopathy and patients tend to have a poor prognosis.
Dilated cardiomyopathy
Nonischemic dilated cardiomyopathy: Delayed-enhancement short-axis cardiac MRI (left image) shows no abnormal enhancement, but there is diffuse concentric dilation of the left ventricular and right ventricular cavities, which is also appreciated on the axial image (right image). Bilateral small pleural effusions are present.
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Dilated cardiomyopathy represents concentric ventricular chamber enlargement with impaired systolic function. Typically, both ventricles are involved.
•Dilatedcardiomyopathycanbeischemicoridiopathicinetiology,andevaluationbyMRI orCTisusefultodeterminetheetiology.Anischemiccausecanbesuggestedbydelayed enhancementinavasculardistributiononMRIorcoronarydiseaseseenonCCTA.
•Catheter angiography is recommended to exclude coronary artery disease in a new diagnosis of dilated cardiomyopathy.
688
•Up to 41% of patients with idiopathic DCM demonstrate abnormal enhancement in a nonischemic distribution in the mid-ventricular wall. The significance of this enhancement in patients with idiopathic DCM is uncertain.
Lipomatous hypertrophy of the interatrial septum
Lipomatous hypertrophy of the interatrial septum: Axial and coronal noncontrast CT demonstrates a focal area of fat attenuation at the interatrial septum, along the right heart border (arrows).
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Lipomatous hypertrophy of the interatrial septum represents proliferation of fatty deposits within the interatrial septum, typically along the lateral right heart border.
•Lipomatous hypertrophy is an incidental finding that does not require treatment. It is important not to mistake it for a cardiac mass. An exceedingly rare differential consideration of a fatty cardiac mass is liposarcoma.
Pericardial disease
Pericardial anatomy
•The pericardium consists of two layers (the visceral and parietal pericardium), which are separated by approximately 40 mL of pericardial fluid.
•The visceral pericardium is too thin to be visualized on imaging. The pericardial apparatus (combination of the visceral and parietal layers and pericardial fluid) measures <1 mm on cadaveric studies and can be seen on CT and MRI, with a normal thickness <2 mm. A pericardial thickness of ≥4 mm on imaging is considered clearly abnormal.
Pericardial effusion
Pericardial effusion:
Short-axis image from a steady state free precession cardiac MRI demonstrates a large pericardial effusion (arrows).
Case courtesy Michael Steigner, MD, Brigham and Women’s Hospital.
689
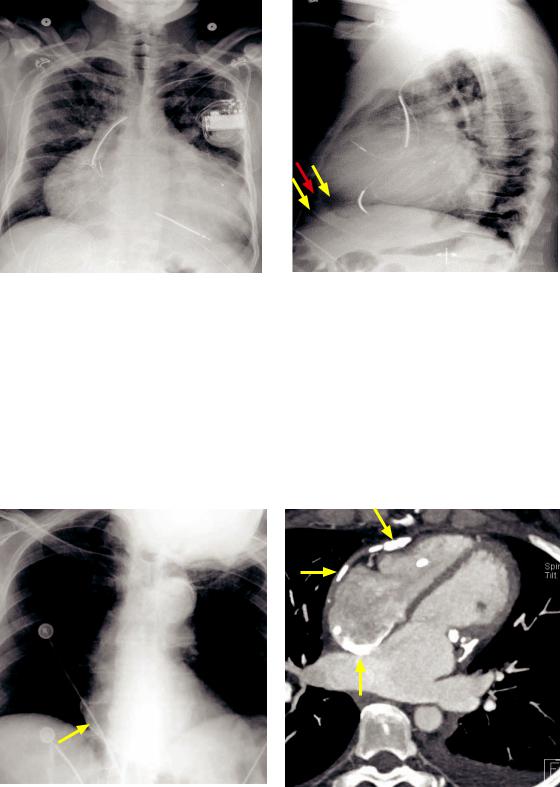
Pericardial effusion and oreo cookie sign (in a different patient): Frontal radiograph (left image) demonstrates a markedly enlarged heart, which is nonspecific but can be seen in pericardial effusion. Lateral radiograph demonstrates the oreo cookie sign with two parallel lucencies (yellow arrows) indicating the epicardial and pericardial fat stripes surrounding a white “filling” indicating the pericardial effusion (red arrow).
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•The primary clinical concern of a pericardial effusion is cardiac tamponade. As little as 100–200 mL of pericardial fluid can impede diastolic filling if it accumulates quickly.
•The classic plain film finding of pericardial effusion is the oreo cookie sign, which represents the parallel lucent epicardial and pericardial fat stripes (the cookies) and the radiopaque pericardial effusion (the white filling).
Pericardial calcification
Pericardial calcification: Chest radiograph shows faint peripheral calcification of the right heart border (arrow). Axial contrast-enhanced CT shows multifocal calcification throughout the pericardium (arrows).
Case courtesy Michael Hanley, MD, University of Virginia Health System.
•Pericardial calcification can be a result of prior pericarditis, most commonly viral or uremic in etiology. Pericardial calcification can be associated with constrictive physiology.
•The main differential of pericardial calcification is myocardial calcification due to old infarct.
690
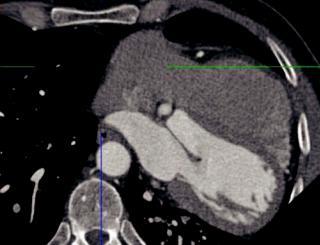
Congenital absence of the pericardium
Congenital absence of the left pericardium:
Axial image from a coronary CT shows an abnormal axis of the heart, which is displaced left-posteriorly into the left hemithorax.
Although a pericardial defect is not directly visualized on this image, the characteristic abnormal cardiac axis should lead one to consider congenital absence of the left pericardium.
Case courtesy Michael Steigner, MD, Brigham and Women’s Hospital.
•Congenital absence of the pericardial is a spectrum of disorders ranging from a focal pericardial defect to complete absence of the right and left pericardium. Total absence of the pericardium is very rare.
•The most common form of pericardial absence involves the left pericardium in the region of the left atrial appendage and adjacent pulmonary artery.
•Patients with partial absence of the pericardium are at risk for herniation of a portion of the heart through the pericardial defect.
•On imaging, a clue to the presence of a pericardial defect is a lucent notch between the aorta and pulmonary artery (sometimes seen on radiography, but generally better appreciated on CT), which represents interposed lung between these vascular structures.
•Defects of the left pericardium cause leftward displacement of the heart, which may in some cases be the only imaging finding.
691

References, resources, and further reading
General References:
Miller, S.W., Abbara, S. & Boxt, L.B. Cardiac Imaging: The Requisites (3rd ed.) Mosby. (2009).
Webb, W.R. & Higgins, C.B. Thoracic Imaging – Pulmonary and Cardiovascular Radiology. Lippincott Williams & Wilkins. (2005).
Aorta:
Agarwal, P.P., Chughtai, A., Matzinger, F.R.K. & Kazerooni, E.A. Multidetector CT of thoracic aortic aneurysms. Radiographics, 29(2), 537-52(2009).
Bashir, M.R., Ferral, H., Jacobs, C., McCarthy, W. & Goldin, M. Endoleaks after endovascular abdominal aortic aneurysm repair: management strategies according to CT findings. AJR. Americanjournal of roentgenology, 192(4), W178-86(2009).
Chao, C.P., Walker, T.G. & Kalva, S.P. Natural history and CT appearances of aortic intramural hematoma. Radiographics, 29(3), 791-804(2009).
Gotway, M.B. et al. Imaging findings in Takayasu’s arteritis. American Journal of Roentgenology, 184(6), 1945(2005).
Hayashi, H. et al. Penetrating Atherosclerotic Ulcer of the Aorta: Imaging Features and Disease Concept. Radiographics, 20(4), 995-1005(2000).
Jeudy, J., Waite, S. & White, C.S. Nontraumatic thoracic emergencies. Radiologic clinics of North America, 44(2), 273-93, ix(2006).
Layton, K. & Kallmes, D. Bovine Aortic Arch Variant in Humans: Clarification of a Common Misnomer. American journal of Neuroradiology, 1541-2(2006).
Ledbetter, S., Stuk, J.L. & Kaufman, J.A. Helical (spiral) CT in the evaluation of emergent thoracic aortic syndromes. Radiologic clinics of North America, 37(3), 515-89(1999).
Rakita, D. et al. Spectrum of CT findings in rupture and impending rupture of abdominal aortic aneurysms. Radiographics, 27(2), 497-507(2007).
Cardiac CT:
Heffernan, E.J., Dodd, J.D. & Malone, D.E. Cardiac multidetector CT: technical and diagnostic evaluation with evidence-based practice techniques. Radiology, 248(2), 366-77(2008).
Hoffmann, U. et al. Coronary CT angiography versus standard evaluation in acute chest pain. The New England journal of medicine, 367(4), 299-308(2012).
Kim, S.Y. et al. Coronary Artery Anomalies: Classification and ECG-gated Multi-Detector Row CT Findings with Angiographic Correlation. Radiographics, 26, 317-34(2006).
Litt, H.I. et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. The New England journal of medicine, 366(15), 1393-403(2012).
O’Brien, J.P. et al. Anatomy of the heart at multidetector CT: what the radiologist needs to know. Radiographics, 27(6), 1569-82(2007).
Raff, G.L. et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. Journal of Cardiovascular Computed Tomography, 3(2), 122-36(2009).
Sparrow, P. et al. CT and MRI Imaging Findings in Patients with Acquired Heart Disease at Risk for Sudden Cardiac Death. Radiographics, 29, 805-23(2009).
692
Miscellaneous:
Chen, J.J., Manning, M.A., Frazier, A.A., Jeudy, J. & White, C.S. CT Angiography of the Cardiac Valves: Normal, Diseased, and
Postoperative Appearances. Radiographics, 29, 1393-412(2009).
Marcus, F.I. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation, 121(13), 1533-41(2010).
Cardiac MRI:
Boxt, L.M. Cardiac MRI Imaging: A Guide for the Beginner. Radiographics, 19(4), 1009-25(1999).
Mahrholdt, H., Wagner, A., Judd, R.M., Sechtem, U. & Kim, R.J. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. European Heart Journal, 26(15), 1461-74(2005).
Vogel-Claussen, J. et al. Delayed Enhancement MRI Imaging: Utility in Myocardial Assessment 1. Radiographics, 26(3), 795 (2006).
Pericardium:
Broderick, L.S., Brooks, G.N. & Kuhlman, J. Anatomic Pitfalls of the Heart and Pericardium. Radiographics, 25, 441-53(2005).
693
