
- •Preface
- •Acknowledgments
- •Introduction
- •Cardiac Tissue Engineering
- •Objectives and Scopes
- •Organization of the Monograph
- •Bibliography
- •Introduction
- •The Heart and Cardiac Muscle Structure
- •Myocardial Infarction and Heart Failure
- •Congenital Heart Defects
- •Endogenous Myocardial Regeneration
- •Potential Therapeutic Targets and Strategies to Induce Myocardial Regeneration
- •Bibliography
- •Introduction
- •Human Embryonic Stem Cells
- •Induced Pluripotent Stem Cells
- •Direct Reprogramming of Differentiated Somatic Cells
- •Cardiac Stem/Progenitor Cells
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Basic Biomaterial Design Criteria
- •Biomaterial Classification
- •Natural Proteins
- •Natural Polysaccharides
- •Synthetic Peptides and Polymers
- •Basic Scaffold Fabrication Forms
- •Hydrogels
- •Macroporous Scaffolds
- •Summary and Conclusions
- •Bibliography
- •Biomaterials as Vehicles for Stem Cell Delivery and Retention in the Infarct
- •Introduction
- •Stem Cell Delivery by Biomaterials
- •Cardiac Stem/Progenitor Cells
- •Clinical Trials
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Myocardial Tissue Grafts Created in Preformed Implantable Scaffolds
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Bioreactor Cultivation of Engineered Cardiac Tissue
- •Mass Transfer in 3D Cultures
- •Bioreactor as a Solution for Mass Transfer Challenge
- •Perfusion Bioreactors
- •Inductive Stimulation Patterns in Cardiac Tissue Engineering
- •Mechanotransduction and Physical/Mechanical Stimuli
- •Mechanical Stimulation Induced by Magnetic Field
- •Electrical Stimulation
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Prevascularization of the Patch by Incorporating Endothelial Cells (ECs)
- •The Body as a Bioreactor for Patch Vascularization
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Decellularized ECM
- •Injectable Biomaterials
- •Injectable hydrogels based on natural or synthetic polymers
- •Injectable Decellularized ECM Matrices
- •Mechanism of Biomaterial Effects on Cardiac Repair
- •Immunomodulation of the Macrophages by Liposomes for Infarct Repair
- •Inflammation, Apoptosis, and Macrophage Response after MI
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Evolution of Bioactive Material Approach for Myocardial Regeneration
- •Bioactive Molecules for Myocardial Regeneration and Repair
- •Injectable Systems
- •Sulfation of Alginate Hydrogels and Analysis of Binding
- •Injectable Affinity-Binding Alginate Biomaterial
- •Summary and Conclusions
- •Bibliography
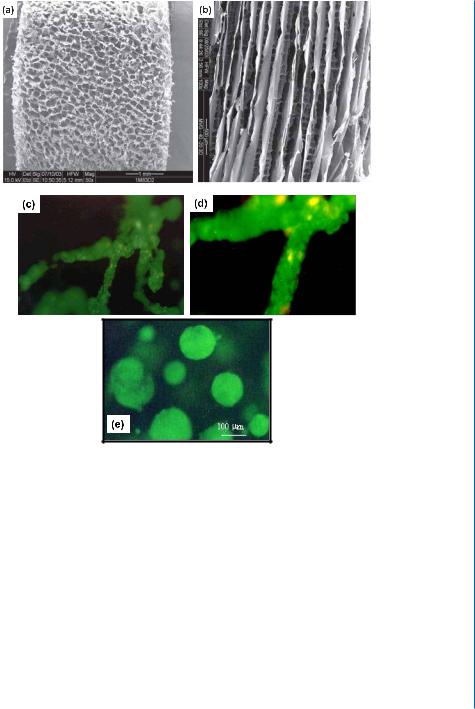
4.5. SUMMARY AND CONCLUSIONS 49
Figure 4.3: Alginate scaffold porosity affects cell behavior and tissue morphology. Depending on the freezing regime, the scaffolds can be prepared with isotropic (A) or anisotropic (B) pore structure. The pore architecture influences cell organization in the scaffold: endothelial cells cultivated in anisotropic alginate scaffolds (C, magnified in D), and C3A (human hepatocyte cell line) spheroids grown in isotropic alginate scaffolds (E).
4.5SUMMARY AND CONCLUSIONS
This chapter provided an overview of the biomaterials used in tissue engineering. It presented the basic criteria for material selection and design, the type of natural and synthetic polymers in use and their advantages/drawbacks, as well as scaffold types and their fabrication methodology. The summary is not exhaustive but focuses on those concepts that have given or are expected to

50 4. BIOMATERIALS – POLYMERS, SCAFFOLDS, AND BASIC DESIGN CRITERIA
give significant input to a better understanding of the biomaterials and their application in various strategies of cardiac tissue engineering and regeneration.

BIBLIOGRAPHY 51
BIBLIOGRAPHY
[1]Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. DOI: 10.1126/science.8493529 41
[2]Williams DF. The Williams Dictionary of Biomaterials: Liverpool University Press; 1999. 42
[3]Segers VF, Lee RT. Local delivery of proteins and the use of self-assembling peptides. Drug discovery today. 2007;12:561–8. DOI: 10.1016/j.drudis.2007.05.003 44, 47
[4]Pok S, Jacot JG. Biomaterials advances in patches for congenital heart defect repair. J Cardiovasc Transl Res. 2011;4:646–54. DOI: 10.1007/s12265-011-9289-8 44
[5]Haraguchi Y, Shimizu T, Yamato M, Okano T. Regenerative therapies using cell sheet-based tissue engineering for cardiac disease. Cardiol Res Pract. 2011;2011:845170.
DOI: 10.4061/2011/845170 44
[6]Drury JL, Dennis RG, Mooney DJ. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187–99. DOI: 10.1016/j.biomaterials.2003.10.002 44
[7]Tous E, Purcell B, Ifkovits JL, Burdick JA. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res. 2011;4:528–42. DOI: 10.1007/s12265-011-9291-1 44
[8]Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. J R Soc Interface. 2012;9:1–19. DOI: 10.1098/rsif.2011.0301 44, 45
[9]Allison DD, Grande-Allen KJ. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12:2131–40. DOI: 10.1089/ten.2006.12.2131 44
[10]Akhyari P, Kamiya H, Haverich A, Karck M, Lichtenberg A. Myocardial tissue engineering: the extracellular matrix. Eur J Cardiothorac Surg. 2008;34:229–41.
DOI: 10.1016/j.ejcts.2008.03.062 45, 47
[11]Al-Shamkhani A, Duncan, R. Radioiodination of alginate via covalently-bound tyrosinamide allows monitoring of its fate in vivo. Journal of Bioactive and Compatible Polymers. 1995;10:4–
13.DOI: 10.1177/088391159501000102 45
[12]Prestwich GD, Kuo JW. Chemically-modified HA for therapy and regenerative medicine. Current pharmaceutical biotechnology. 2008;9:242–5. DOI: 10.2174/138920108785161523
[13]Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release. 2011;155:193–9.
DOI: 10.1016/j.jconrel.2011.04.007 46

52BIBLIOGRAPHY
[14]Masuda S, Shimizu T, Yamato M, Okano T. Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev. 2008;60:277–85. DOI: 10.1016/j.addr.2007.08.031 47
[15]Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24:2309–16. DOI: 10.1016/S0142-9612(03)00110-8 47
[16]Giraud MN, Armbruster C, Carrel T, Tevaearai HT. Current state of the art in myocardial tissue engineering. Tissue Eng. 2007;13:1825–36. DOI: 10.1089/ten.2006.0110 47
[17]Wang F, Guan J. Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy. Adv Drug Deliv Rev. 2010;62:784–97. DOI: 10.1016/j.addr.2010.03.001 47
[18]Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. Myocardial tissue engineering: a review. J Tissue Eng Regen Med. 2007;1:327–42.
DOI: 10.1161/01.RES.0000196562.73231.7d 47
[19]Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–9. DOI: 10.1089/107632704323061762 47
[20]Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–60. DOI: 10.1016/j.jacc.2004.04.040 47
[21]Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–9. DOI: 10.1016/j.jacc.2005.04.056 47
[22]Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. DOI: 10.1161/CIRCULATIONAHA.107.727420 47
[23]Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009;54:1014–23. DOI: 10.1016/j.jacc.2009.06.010 47
[24]Wang T, Wu DQ, Jiang XJ, Zhang XZ, Li XY, Zhang JF, et al. Novel thermosensitive hydrogel injection inhibits post-infarct ventricle remodelling. Eur J Heart Fail. 2009;11:14–9.
DOI: 10.1093/eurjhf/hfn009 47
[25]Weigel T, Schinkel G, Lendlein A. Design and preparation of polymeric scaffolds for tissue engineering. Expert review of medical devices. 2006;3:835–51. DOI: 10.1586/17434440.3.6.835 48

BIBLIOGRAPHY 53
[26]Shapiro L, Cohen S. Novel alginate sponges for cell culture and transplantation. Biomaterials. 1997;18:583–90. DOI: 10.1016/S0142-9612(96)00181-0 48
[27]Zmora S, Glicklis R, Cohen S. Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials. 2002;23:4087–94.
DOI: 10.1016/S0142-9612(02)00146-1 48
