
- •Preface
- •Contents
- •Procedures, Assays, and Normal Values
- •Normal Cells of the Blood and Hematopoietic Organs
- •The Individual Cells of Hematopoiesis
- •Bone Marrow: Cell Composition and Principles of Analysis
- •Abnormalities of the White Cell Series
- •Predominance of Mononuclear Round to Oval Cells
- •Prevalence of Polynuclear (Segmented) Cells
- •Erythrocyte and Thrombocyte Abnormalities
- •Hypochromic Anemias
- •Normochromic Anemias
- •Hyperchromic Anemias
- •Erythrocyte Inclusions
- •Thrombocyte Abnormalities
- •Cytology of Organ Biopsies and Exudates
- •Lymph Node Cytology
- •Branchial Cysts and Bronchoalveolar Lavage
- •Cytology of Pleural Effusions and Ascites
- •Cytology of Cerebrospinal Fluid
- •Introduction to the Physiology and Pathophysiology of the Hematopoietic System
- •Cell Systems
- •Principles of Regulation and Dysregulation in the Blood Cell Series and their Diagnostic Implications
- •Procedures, Assays, and Normal Values
- •Taking Blood Samples
- •Erythrocyte Count
- •Hemoglobin and Hematocrit Assay
- •Calculation of Erythrocyte Parameters
- •Red Cell Distribution Width (RDW)
- •Reticulocyte Count
- •Leukocyte Count
- •Thrombocyte Count
- •Significance of the Automated Blood Count
- •Bone Marrow Biopsy
- •Lymph Node Biopsy and Tumor Biopsy
- •Step-by-Step Diagnostic Sequence
- •The Individual Cells of Hematopoiesis
- •Eosinophilic Granulocytes (Eosinophils)
- •Basophilic Granulocytes (Basophils)
- •Monocytes
- •Lymphocytes (and Plasma Cells)
- •Megakaryocytes and Thrombocytes
- •Bone Marrow: Medullary Stroma Cells
- •Abnormalities of the White Cell Series
- •Predominance of Mononuclear Round to Oval Cells
- •Reactive Lymphocytosis
- •Relative Lymphocytosis Associated with Granulocytopenia (Neutropenia) and Agranulocytosis
- •Monocytosis
- •Acute Leukemias
- •Neutrophilia without Left Shift
- •Reactive Left Shift
- •Osteomyelosclerosis
- •Elevated Eosinophil and Basophil Counts
- •Clinically Relevant Classification Principle for Anemias: Mean Erythrocyte Hemoglobin Content (MCH)
- •Hypochromic Anemias
- •Iron Deficiency Anemia
- •Hypochromic Infectious or Toxic Anemia (Secondary Anemia)
- •Hypochromic Anemia with Hemolysis
- •Normochromic Anemias
- •Normochromic Hemolytic Anemias
- •Cytomorphological Anemias with Erythrocyte Anomalies
- •Bone Marrow Aplasia
- •Hyperchromic Anemias
- •Erythrocyte Inclusions
- •Hematological Diagnosis of Malaria
- •Thrombocyte Abnormalities
- •Thrombocytopenia
- •Lymph Node Cytology
- •Sarcoidosis and Tuberculosis
- •Non-Hodgkin Lymphoma
- •Metastases of Solid Tumors in Lymph Nodes or Subcutaneous Tissue
- •Branchial Cysts
- •Cytology of Pleural Effusions and Ascites
- •Cytology of Cerebrospinal Fluid
- •References
- •Index

140 Erythrocyte and Thrombocyte Abnormalities
Normochromic Anemias
Anemias where red cell hemoglobinization is normal (26–32 pg/dl, equivalent to 1.61–1.99 fmol/l) and average MCVs are normal (77–100 fl) can broadly be explained by three mechanisms: a) acute blood loss with sufficient metabolic reserves remaining; b) elevated cell turnover in which iron is reused as soon as it becomes free, so that hypochromia does not arise (this is typical of almost all hemolytic anemias except thalassemias; see p. 138); and c) suppression of cell production under conditions of normal iron supply (this is the group of hypoplastic–aplastic anemias, which have a variety of causes).
— In cases of acute blood loss: clinical findings, occult blood?
—In hemolytic cases: reticulocytes !, haptoglobin ", possibly bilirubin
!.
—In bone marrow suppression: e.g. aplastic anemia, reticulocytes ".
Normochromic Hemolytic Anemias
Hemolytic anemias result from a shortened erythrocyte life span with insufficient compensation from increased erythrocyte production (Table
24).
Usually, hematopoiesis in the bone marrow is increased in compensation, and, depending on the course of the disease, may make up for the accelerated cell degradation for all or some of the time by recycling the iron as it becomes free.
Accordingly, counts of the young, newly emerged erythrocytes (reticulocytes) are always raised, and usually sporadic normoblasts are found. Anemia proper often becomes apparent only in a “crisis” with acute, accelerated cell degradation, and reticulocyte counts increased up to more than 500%.
A common cellular phenomenon after extended duration of hemolytic anemia is the manifestation of macrocytic hypochromic disorders (p. 150), because the chronic elevation of hematopoietic activity can exhaust the endogenous folic acid reserves (pernicious anemia).
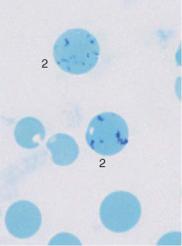
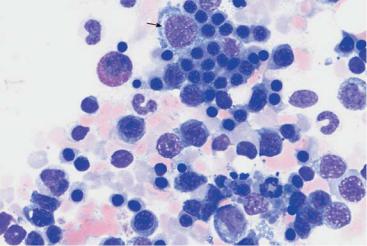
Bone marrow analysis shows both relative and absolute increases in erythropoietic activity: among the red cell precursors, in acute severe hemolysis the more immature forms often predominate more than in normal bone marrow, and in chronic hemolysis the maturer forms do (orthochromatic normoblasts). In addition, the normoblasts in hemolytic bone marrow often are markedly clustered (Fig. 48), whereas in normal bone marrow they are more evenly dispersed (Fig. 18).

Consistently elevated “young” erythrocytes (reticulocytes) suggest hemolysis
a 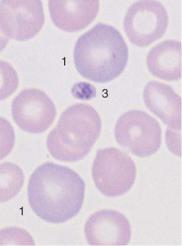
 b
b
 c
c
Fig. 48 Hemolytic anemia. a and b Newly formed erythrocytes appear as large, polychromatic erythrocytes (1) after Pappenheim staining (a); supravital staining (b) reveals spot-like precipitates (reticulocyte = 2). Thrombocyte (3). c Bone marrow cells in hemolytic anemia at low magnification: increased hematopoiesis with cell clusters. Orthochromatic erythroblasts predominate. A basophilic erythroblast shows loosened nuclear structure (arrow), a sign of secondary folic acid deficiency.
141

142 Erythrocyte and Thrombocyte Abnormalities
Table 24 Causes of the most common hemolytic anemias
Special morphological |
Further advanced |
features of erythrocytes |
diagnostics |
|
|
Causes within the erythrocytes (corpuscular hemolyses)
Hereditary
! Membrane abnormalities |
|
|
– Spherocytosis (see p. 144) |
– Small spherocytes |
Osmotic resistance |
– Elliptocytosis |
– Elliptocytes |
|
! Hemoglobin abnormalities |
|
|
– Thalassemia (see p. 138) |
– Target cells |
Hemoglobin electro- |
– Sickle cell anemia (see |
– Sickle cells |
phoresis |
p. 144) |
|
|
– Other rare hemoglobin- |
|
|
related disorders |
|
|
! Enzyme defects |
|
|
– Glucose-6-phosphate |
– Possibly Heinz bodies |
Enzyme tests |
dehydrogenase |
– Macrocytes |
|
– Pyruvate kinase and |
|
|
many others |
|
|
Acquired |
|
|
! Paroxysmal nocturnal |
|
Sucrose hemolysis |
hemoglobinuria |
|
test, absence of CD 55 |
! Zieve syndrome |
|
(DAF) and CD 59 |
– Foam cells in the bone |
MIRL (membrane |
|
|
marrow |
inhibitor of reactive |
|
|
lysis) |
|
||
Causes outside the erythrocytes (extracorpuscular hemolyses) |
||
Biosynthesis of antibodies |
|
|
! Isoantibodies (fetal erythro- |
|
Rh serology |
blastosis, transfusion events) |
|
|
! Warm autoantibodies |
|
Coombs test |
! Cold autoantibodies |
– Autoagglutination |
Coombs test, |
|
|
Cold agglutination |
! Chemical-allergic antibodies |
|
titer |
|
|
|
(e. g., cephalosporin, methyl- |
|
|
dopa) |
|
|
Physical or chemical noxae |
– Partially Heinz bodies |
|
(e. g., after burns, heart valve |
|
|
replacement; heavy metal |
|
|
exposure, animalor plant- |
|
|
derived poisons) |
|
|
Microangiopathic hemolysis in |
– Schizocytes, fragmen- |
Thrombocytes " |
hemolytic-uremic syndrome, |
tocytes (see p. 143) |
Liver, kidney |
thrombotic-thrombocytopenic |
|
|
purpura, bone marrow carci- |
|
|
noses |
|
|
Infection-related noxae (e. g. |
– For malaria pathogen |
Demonstration of |
influenza, salmonella infection, |
(see p. 158) |
pathogen |
malaria) |
|
|
Hypersplenism, e. g. lymphatic |
|
Cause of |
system disease, infections with |
|
splenomegaly |
splenomegaly, portal hyperten- |
|
|
sion |
|
|
|
|
|

Distribution pattern and shape of erythrocytes can be relevant in the diagnosis of hemolysis
a 
b 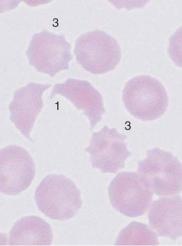
 c
c
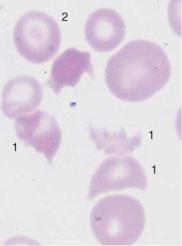
Fig. 49 Autoagglutination and fragmentocytes. a Clumps of erythrocytes. If this is the picture in all regions of the smear, an artifact is unlikely and serogenic (auto)agglutination should be suspected (in this case due to cryoagglutinins in mycoplasmic pneumonia). Thrombocytes are found between the agglutinated erythrocytes. b and c Conspicuous half-moon and egg-shell-shaped erythrocytes: fragmentocytosis in microangiopathic hemolytic anemia. Fragmentocytes (1), target cell (2), and echinocytes (3) (this last has no diagnostic relevance).
143
